Effect of the ester group in side chain of polycarboxylate superplasticizer on the cement hydration process
In this paper, the effect of the presence of the ester group in side chain of polycarboxylate superplasticizer (PC) on the cement hydration process was discussed. PC without the ester group was synthesized with acrylic acid (AA) and isoprenyl polyoxyethylene (IPEG), and PC2 with the ester group was synthesized with acrylic acid (AA), hydroxyethyl acrylate (HEA) and IPEG. The chemical structure of copolymer was investigated by FTIR and GPC. The stability of the ester group in side chain was tested by FTIR, pH value and 1H-NMR, and the adsorption amount was measured by TOC. Then the hydration process of cement paste with additional copolymer was examined by hydration heat and XRD. The results show that: under the alkaline environment in the cement hydration process, the ester group in side chain can be decomposed into the carboxyl group and glycol. And the presence of the ester group in side chain can weaken the initial adsorbing ability and obviously enhance the later adsorbing ability, because of the increase of the carboxyl group originating from the decomposition of the ester group. PC with the ester group has less initial dispersion, and the decomposition of the ester group can enhance its later dispersion, which is the main reason for the fluidity increase of cement paste over time. It is suggested that PC containing the ester group in side chain can be used to delay the cement hydration and improve the fluidity loss of fresh cement paste and concrete.
1 Introduction
In recent years, the cement-based materials have been significantly improved by high performance superplasticizer [1-4], which is shown as the high fluidity of the fresh concrete [5-7] and the low porosity of hardened concrete by using less water [8-9]. Polycarboxylate superplasticizer (PC), which consists of one main chain and many side chains, is well known as the most efficient superplasticizer [10-12]. Steric hindrance, provided by the long side chain such as polyethylene oxide (PEO), is the main reason for its high efficient dispersion [13-14]. The length of the long side...
1 Introduction
In recent years, the cement-based materials have been significantly improved by high performance superplasticizer [1-4], which is shown as the high fluidity of the fresh concrete [5-7] and the low porosity of hardened concrete by using less water [8-9]. Polycarboxylate superplasticizer (PC), which consists of one main chain and many side chains, is well known as the most efficient superplasticizer [10-12]. Steric hindrance, provided by the long side chain such as polyethylene oxide (PEO), is the main reason for its high efficient dispersion [13-14]. The length of the long side chain, which is determined by molecular weight of PEO, is proved as the main factor for initial dispersion [15-17]. And the short side chain containing the carboxyl group and sulfonic acid group plays an important role in the adsorbing process, which shows that the adsorbing ability of PC depends on the density of the carboxyl group in short side chain [18-21]. All this helps to understand the relationship between structure and performance of PC.
Adsorption is the first step for the reaction between PC and cement particles, and is of great importance to the performance of PC [22-23]. The fast-adsorbed PC can provide the stronger initial dispersion to disperse the cement particles, but may lead to fluidity loss over time. While the slow-adsorbed PC have less initial dispersion, but the dispersion can be increased later, which may show better maintaining dispersion [19, 24]. Therefore, how to control the adsorbing process is critical for dispersion and maintaining dispersion of PC [25-27]. Generally, it is feasible to adjust the grating density of the short side chain mainly including the carboxyl group to improve the adsorbing ability of PC. However, the neutral monomer, such as the ester group, can reduce the anion effect of the side chain and also preempt the position of the carboxyl group in side chain to reduce the grafting density of the carboxyl group. In this way, the adsorbing speed can be slowed down. The presence of the ester group probably brings about the significant difference from other types of PC without the ester group in side chain.
In this paper, two types of PC, including PC1 without the ester group and PC2 with the ester group, were discussed. The molecular structure of PC was investigated by FTIR and GPC. The stability of PC2 with the ester group in side chain was tested by FTIR, pH value and 1H-NMR, and the adsorption amount was measured by TOC. Then the hydration process of cement paste with PC was discussed with hydration heat and XRD. Finally, the dispersion of PC was tested in fresh cement paste and concrete. It is expected that the results can suggest a new method to control the adsorption process and improve the maintaining dispersion of the superplasticizer system in fresh cement paste and concrete.
2. Experimental methods
2.1 Materials
2.1.1 Cement
An ordinary Portland cement (42.5, Wuhan Yadong Cement Co., Ltd.) meeting the requirements of the Chinese GB8076 standard was used. The chemical composition of cement was showed in Table 1. The content of CaSO4 in cement is 4.2 %. The density is 3.10 g/cm3, and the specific surface area is 350 m2/kg.
2.1.2 Polycarboxylate superplasticizer
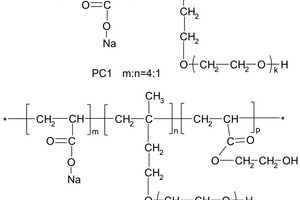
PC1 was synthesized with acrylic acid (AA) and isoprenyl polyoxyethylene (IPEG), and the molar ratio was 4:1. PC2 was synthesized with AA, hydroxyethyl acrylate (HEA) and IPEG, and the molar ratio was 1:3:1. A schematic diagram of the chemical structure of PC1 and PC2 is shown in Figure 1.
2.2 Test methods
2.2.1 Adsorption measurement
A series of PC solutions with different concentration were prepared in advance. Cement (1.0 g) was mixed with the PC solution (20.0 g) and stirred for 5 min or 60 min, and then the mixture was separated by centrifugation at 3000 r/min for 4 min. The upper supernatant was prepared for measurement. The organic carbon content in the upper supernatant was measured by Total Organic Carbon Analyzer (TOC, Multi N/C 2100, made in Jena/Germany). And the results of TOC were used to calculate the adsorption amount of PC as follows:
Adsorption amount (mg/g-cement) = V (C0-C)/m⇥(1)
C0 is the initial concentration (g/L) of PC before adsorbing, and C is the residual concentration (g/L) after adsorbing. V is volume of the solution (mL). m is the mass of the cement (g).
Measurements were generally repeated three times and the average was the result. All operation was made at 25 °C.
2.2.2 pH value
PC2 (100 g, 20 wt %) and NaOH (100 g, 0.2 g/L) were mixed together and stirred quickly, and then the pH value was tested by the pH meter. After the pH value didn’t vary at all, hydrochloric acid was used to adjust the pH value to 7.0. And then, the solution was dried in a vacuum drier at 60 °C. The solid was prepared for measurement of 1H-NMR and FTIR.
2.2.3 FTIR
The FTIR spectra of the samples were recorded with the Nexus Fourier-transform infrared spectrometer (Nexus, made by Thermo Nicolet, USA) by coating purify anhydrous PC on KBr pellets. In order to test the hydrophobic aliphatic group (-CH-, -CH2-, -CH3), ester group (-COOCH2-), sodium carboxylate (-COONa) and polyoxyethylene group (-CH2-O-CH2-), FTIR spectra for PC were taken over the whole spectral range (4000-400 cm-1).
2.2.4 1H-NMR
The 1H-NMR spectra were obtained by Nuclear Magnetic Resonance (AVANCE III 400WB, made by Bruker, Germany) at room temperature. From the analysis of 1H-NMR, we could infer whether the ester group (-COOCH2CH2OH) could be decomposed into carboxyl group and glycol (HOCH2CH2OH).
2.2.5 GPC
The molecular weight (Mw, Mn) and poly-dispersity index (PDI) were determined at 25 °C by Gel Permeation Chromatography (GPC, Agilent 1260-RID, made in the USA). The eluent is 0.1 mol/l NaNO3; current speed is 1.0 ml/min. The concentration of the sample is 0.5 wt%.
2.2.6 Fluidity cement paste
A certain amount of PC was added to water in advance. And then fresh cement paste was prepared with a water/cement weight ratio of 0.29:1 (water: 87 g, cement: 300 g), meeting the requirements of Chinese standard GB 8077-2008. A flow cone (60 mm height, 36 top diameter, 60 mm bottom diameter) specified in Chinese standard GB 8077-2000 was filled up with fresh cement paste on a glass plat, and then the cone was removed. The maximum diameter of the spread sample and the maximum width perpendicular to the maximum diameter were measured. The average of these two values was defined as the fluidity value, which was used to compare the dispersion of PC. All operations were conducted at 25 °C.
2.2.7 XRD
Cement paste was prepared with a water/cement weight ratio of 0.35:1. And the samples were cured in the > 90 % R.H. chamber at 20 ± 1 °C until designated ages. After 1 d or 7 d, they were treated with anhydrous alcohol in order to end their hydration, and then broken into small pieces and powder. The powder samples were tested by X-ray Diffractometer (XRD, D/Max-RB, made by Rigaku, Japan) with Cu (Kα) radiation and a current of (40 mA, 40 kV), at a speed of 4 °/min and a step of 0.02° within the range from 5 to 70°.
2.2.8 Hydration heat
The solution with PC and cement was mixed together with a water/cement weight ratio of 0.35:1. Hydration heat of cement paste was tested by micro-calorimeter (TAM AIR, made in the USA).
3 Results and discussion
3.1 Characterization of copolymers
Copolymers were tested by FTIR and GPC. The solid content, pH value, molecular weight (Mw, Mn) and poly-dispersity index (PDI) are shown in Table 2. The results show that Mw and Mn are different between PC1 and PC2. The PDI describes the uniformity of a polymer with respect to molecular weight distribution. PC1 and PC2 possess the same type side chains of PEO, and have a narrow and similar molecular weight distribution. The ratio of the long side chain of PEO and the carboxyl group of PC1 is 1:4, and the ratio of long side chain of PEO, the carboxyl group and the ester group of PC2 is 1:1:3. The density of carboxyl group in side chain of PC1 is about four times as much as that of PC2.
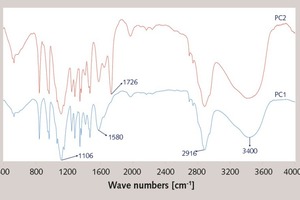
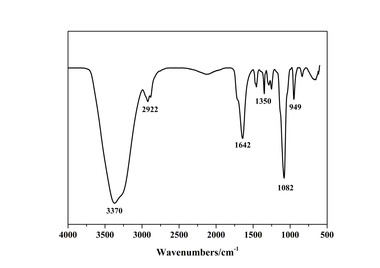
The FTIR analysis was taken over selected spectral ranges for the specific frequencies of stretching vibration for the aliphatic group (-CH-, -CH2-,
-CH3, 3200–2500 cm-1), ester group (-COOCH2-, 1700-1750 cm-1), sodium carboxylate (-COONa, 1550-1650 cm-1), hydroxyl (-OH, 3350-3450 cm-1)
and polyoxyethylene group (-CH2-O-CH2-, 1100-1112 cm-1). FTIR spectra of PC1 and PC2 are shown in Figure 2. The peaks of aliphatic group (-CH-,
-CH2-, -CH3, 2916 cm-1), sodium carboxylate
(-COONa, 1580 cm-1) and polyoxyethylene group (-CH2-O-CH2-, 1106 cm-1) can be found in the spectra of both PC1 and PC2, while the peak of ester group (-COOCH2-, 1726 cm-1) can only be found in the spectrum of PC2. It indicates that the ester group exists in PC2 while no ester group exists in PC1.
3.2 Stability of the ester group
It is well known that the pH value can reach at about 13 in the cement hydration process. Under the alkaline environment, the ester group in side chain of PC may be decomposed into the carboxyl group and alcohol, as is shown as follows:
-COOCH2CH2OH + NaOH ➝ -COONa+ HOCH2CH2OH
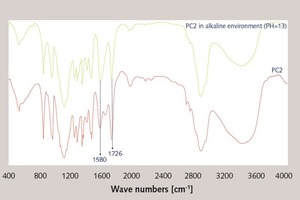
In order to verify the decomposition of the ester group in PC2, the FTIR and 1H-NMR were used to discuss the stability of the copolymer under the alkaline condition (pH = 13).
The FTIR spectra of PC2 and PC2 under the alkaline condition (pH = 13) can be seen in the Figure 3. From the figure, the peak for ester group (-COOCH2-, 1726 cm-1) is reduced and the peak for sodium carboxylate (-COONa, 1580 cm-1) is increased, which illustrates that the ester group has been decomposed into the carboxyl group and forms the sodium carboxylate (-COONa, 1580 cm-1) under the alkaline condition.
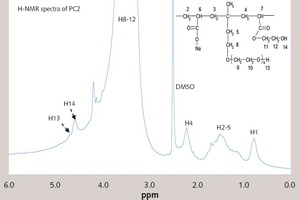
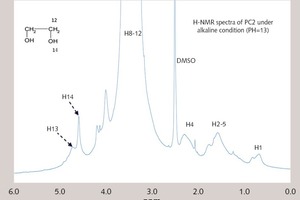
1H-NMR analyses of PC2 and PC2 under the alkaline environment (pH = 13) were summarized in Figure 4. The glycol (HOCH2CH2OH) originating from the decomposition of the ester group can increase the content of the alcohol hydroxyl groups (-OH), which can be seen in the NMR spectra. Hence, the decomposition can be indicated from the difference of 1H-NMR between PC2 and PC2 under the alkaline condition (pH = 13). The peaks of C-CH2-C (δ = 1.0-2.0 ppm), -C-O-CH2- (δ = 3.0~4.0 ppm), -CH3 (δ = 0.8~1.0 ppm) and -OH (δ = 4.50~4.60 ppm) can be seen in the figure. Under the alkaline condition, the peak of -OH (δ = 4.50~4.60) is enhanced, which is mainly caused by the increase of the glycol (HOCH2CH2OH). It indicates that the ester groups (-COOCH2CH2OH) have been decomposed to release the glycol (HOCH2CH2OH). Thus, it is evident that ester group in PC2 can be decomposed in cement hydration process.
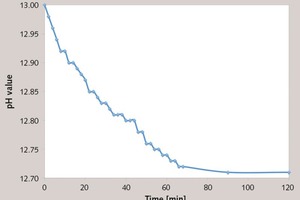
For further proof, the pH value of PC2 under the alkaline condition (pH = 13) was tested. A certain amount of NaOH solution was mixed with the PC solution, and made sure that the initial pH value was about 13.0. The results are summarized in Figure 5. The pH value was decreased within 60 min, and after 60 min, there is little change. It indicates that under the alkaline condition, the ester group can be decomposed, which is also confirmed in FTIR and NMR.
Obviously, these results demonstrate that the ester group in side chain of PC can be easily decomposed into the carboxyl group and organic alcohol in the cement hydration process.
3.3 Adsorption
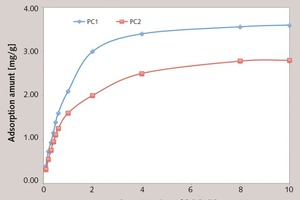
It has been proved that the adsorption is the first step of reaction between PC and the cement particles, and the carboxyl group in side chain is the main adsorbing group of PC. More density of the carboxyl group leads to the fast superficial adsorption [16, 20, 23-24]. Because the density of the carboxyl group in PC1 is larger than that of PC2, it can be inferred that the initial adsorption amount of PC1 is larger than that of PC2, which is in agreement with the results shown in Figure 6.
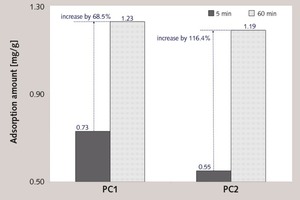
Figure 7 shows the adsorption amount of PC at 5 min and 60 min. No matter 5 min or 60 min, the adsorption amount of PC1 is larger than that of PC2. But there is a significant difference in the increase of the adsorption amount from 5 min to 60 min. The adsorption amount of PC1 is increased by 0.5 mg/g, which is 68 % of adsorption amount at 5 min. while for PC2, it is increased by 0.64 mg/g, which is 116 % of adsorption amount at 5 min. This illustrates that the increase of the adsorption amount of PC1 within 60 min is less than that of PC2, which is closely related to the decomposition of the ester group in side chain. The ester group in fresh cement paste can be decomposed to bring the carboxyl group to increase the grafting density of the carboxyl group, which can significantly improve the superficial adsorbing ability to increase the adsorption amount of PC2 within 60 min.
3.4 Impact on cement hydration
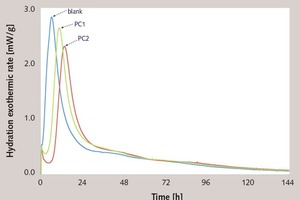
To discuss the retarding effect of PC, the hydration heat was tested using the cement paste (w/c ratio = 0.35). The results are shown in Figure 8 and Table 3. It is demonstrated that both PC1 and PC2 delay the hydration of cement paste, while PC2 has stronger retarding effect than that of PC1, which is also indicated from the hydration heat of 1 d and 7 d shown in Table 3.
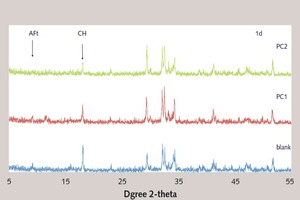
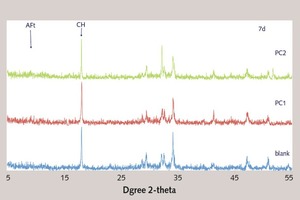
The XRD results are shown in Figure 9. From the XRD analysis of the early hydration products, the retarding effect of PC1 and PC2 on cement hydration can be indicated from the decrease of the characteristic diffraction peak of CH. As shown in the figure, it can be seen that the peak of CH with PC1 is higher than that of PC2. And it indicates that PC2 has stronger retarding effect than PC1, which is the same result as the analysis of hydration heat. However, for 7 d, the peak of CH has little difference between blank and PC. It can be inferred that PC1 and PC2 have little retarding effect on later cement hydration.
The retarding effect of PC depends on the adsorption behavior of the superplasticizer. At first, when cement and superplasticizer solution are mixed together, the superplasticizer adsorbs onto the surface of cement particles to disperse the particles. Simultaneously, when water contacts with the cement surface, the hydration activity points are brought about and the ions are released into solution. And then, the hydration products are formed to enwrap the adsorbed superplasticizer, and the new points are produced for the future adsorption of superplasticizer in solution. Superplasticizer, which has a fast adsorbing speed at the beginning, can be easily enwrapped and consumed by the hydration products in the first few minutes. In this way, there are fewer polymers in solution for the future adsorption onto the surface of new hydration products to control the growth of hydration products. However, those superplasticizers, which have a slow adsorbing speed at the initial time, can reduce the consumption of the early hydration products, and there are more polymers in the solution to supply the later adsorption to restrain the growth of the hydration products.
At the very beginning, compared with PC1, less PC2 adsorbs onto the surface of cement minerals, and more PC2 exists in solution waiting for the later adsorption. At the same time, the hydration products are produced and the pH value of the solution is increased. A few minutes later, the adsorption ability of PC2 is enhanced because of the decomposition of the ester group, and more PC2 can adsorb onto surface of the hydration products to restrain the growth of the products. In this way, the presence of the ester group in side chain of PC provides a better retarding effect on early cement hydration.
3.5 Impacts on fluidity of cement paste
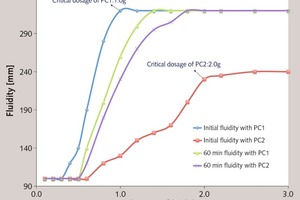
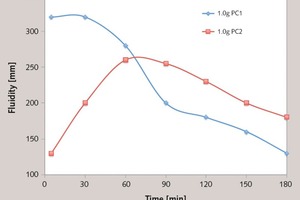
The fluidity of the cement paste with varied dosages of PC is summarized in Figure 10. With the increasing dosage of PC, the initial fluidity and the 60 min fluidity are increased. It can be seen that the fluidity with PC1 is increased more steeply than that of PC2 at the low dosage, and there is a maximum fluidity at a certain dosage. For PC1, it is about 320 mm at the dosage of 1.0 g, and that of PC2 is about 230 mm at the dosage of 2.0 g. It indicates that the initial dispersion of PC1 is much stronger than that of PC2.
As shown in Figure 11, with PC1, the 60 min fluidity is less than the initial fluidity. However, with PC2, the result is completely different: the 60 min fluidity is larger than the initial fluidity. We are particularly interested in the reason why the fluidity of the cement paste with PC1 is firstly increased and then reduced. It is known that the change in fluidity over time is determined by two factors: the cement hydration and the adsorption of superplasticizer. Specifically, the water and superplasticizer are consumed in early cement hydration, and formation of the hydration products reduces the fluidity. In addition, the hydration products can wrap the superplasticizer and weaken its dispersion. If superplasticizer in the solution were accelerated to adsorb onto surface of particles and supply the consumed section, the fluidity would be increased, which is shown as the improvement in the fluidity loss. If the superplasticizer in the solution cannot compensate the consumed parts, the fluidity loss would be exhibited. Therefore, the enhanced adsorbing ability over time is responsibly for the fluidity increase of the cement paste with the addition of PC with ester group in side chain.
3.6 Application results of PC used in concrete
The concrete is made of cement, sand, crushed stone and water according to the weight ratio of 400:760:1075:165.
The results including slump and compressive strength are shown in Table 4. From the table, it can be seen that the slump of fresh concrete with PC1 is very different from that of PC2. With PC1, the fresh concrete has a little slump loss within 1 h, and it is reduce to 70 mm at 3 h with a dosage of 0.5 %. However, when the dosage of PC2 is 0.5 %, the slump is increased slightly within 1 h, and there is nearly no slump loss within 3 h. With mixture of PC1 and PC2, the slump loss is also obviously improved. It indicates that PC with the ester group in side chain can significantly improve the slump loss of fresh concrete. In addition, the compressive strength of concrete with PC1 at the age of 1 d is greater than that of PC2, but there is little difference at 7 d or 28 d. It indicates that PC2 merely slows down the early hydration of cement, but it has little retarding effect on the later cement hydration, which remains in agreement with the analysis of XRD and hydration heat.
From the above discussion, it is easy to find that those PCs containing the ester group in side chain can be used to reduce the fluidity loss. It also suggests a possibility that different types of PC can be used together to improve the workability of high performance concrete.
4 Conclusion
Under the alkaline condition of cement hydration, the ester group in side chain of PC can be decomposed into the carboxyl group and glycol.
The initial adsorption ability of PC can be weakened with the presence of the ester group in side chain because of the less density of the carboxyl group in side chain, and the later adsorption ability and the retarding effect can be obviously enhanced, because of the decomposition of the ester group to increase the density of the carboxyl group.
PC with ester group has less initial dispersion, but the later dispersion can be enhanced due to the decomposition of the ester group, which is the main reason for the fluidity increase of cement paste over time. It is suggested that PC containing ester group in side chain can be used to delay the cement hydration and improve the fluidity loss of fresh cement paste and concrete.
Acknowledgment
We are grateful for the financial support of the National Natural Science Foundation of China (51408448) and the science and technology support of Hubei Province of China (2015BAA084).

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.