Physical-mechanical properties and hydration microstructure of slag powder/cement system with different nanoparticles
Nano-silica (NS) and self-made nano-calcium silicate hydrate (NC) were mixed into the slag powder/cement system, and the effects of the two on the physical-mechanical properties and hydration microstructure of the slag powder/cement system were compared. Results show that the incorporation of NS and NC can shorten the setting time, promote the early hydration of the composite binders and greatly improve the early strength of the matrix. However, since the activity of NS is higher than that of NC, the improvement effect of NS on 1 d, 3 d and 7 d strength of the slag powder/cement system is higher than that of NC. At 28 d, both NS and NC had no significant effect on the matrix strength. The pozzolanic effect of NS and the nucleation effect of NC can provide more seed crystals for the hydration of cementitious materials, and the generated hydration products can fill in the pores between different size particles and improve the early microstructure, which in turn increases the early strength of the slag powder/cement system.
1 Introduction
Cement is the most used cementitious material in engineering construction in China. Its hardened paste is loose and porous, and the erosive substances can easily enter through the pores and cause deterioration of the matrix. Some studies have shown that adding a certain amount of mineral admixtures into cement can effectively refine the pore structure [1], increase the compactness [2] and improve the durability of cement-based materials [3, 4]. However, the adding of mineral additives often causes a reduction in the early strength of cement-based materials [5], which is...
1 Introduction
Cement is the most used cementitious material in engineering construction in China. Its hardened paste is loose and porous, and the erosive substances can easily enter through the pores and cause deterioration of the matrix. Some studies have shown that adding a certain amount of mineral admixtures into cement can effectively refine the pore structure [1], increase the compactness [2] and improve the durability of cement-based materials [3, 4]. However, the adding of mineral additives often causes a reduction in the early strength of cement-based materials [5], which is unfavorable for their engineering applications.
As an emerging and rapidly developing material science, both nanotechnology and nanomaterial have gradually begun to be applied in engineering fields [6, 7]. Studies have shown that nano-silica (NS) can improve the early strength of cement-based materials [8] and increase the chemically bound water content of the matrix [9], and together with slag powder can also synergistically increase the resistance to chloride erosion of the matrix [10]. Moreover, many existing researches have confirmed that nano-calcium silicate hydrate (NC) and C-S-H gels have similar structures [11], and their incorporation into cement can provide additional nucleation sites for cement hydration [12], shorten setting time [13], increase early strength and improve the impermeability of the matrix [13, 14]. In short, both NS and NC can improve the early strength of cement-based materials, but the current research has not compared the effect of the two on performance improvement of the slag powder/cement system under the same experimental conditions.
This paper describes how NS and NC were mixed into the slag powder/cement system, and how the effects of the two on the physical and mechanical properties of the matrix were compared by measuring the fluidity, setting time and compressive strength, and the formation mechanism of the hydration microstructure of the slag powder/cement system by the two nanoparticles was revealed by resistivity, X-ray diffraction (XRD), differential scanning calorimetry-thermogravimetry (DSC-TG), and scanning electron microscopy (SEM).
2 Experiment
2.1 Raw materials
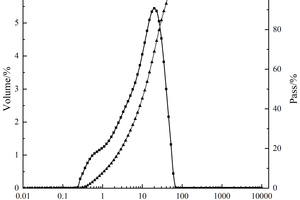
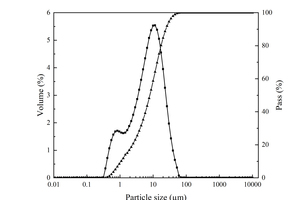
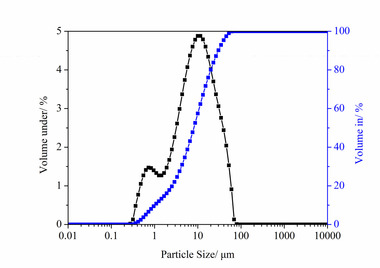
The raw materials used in the experiments included PO·42.5 cement, S95 slag powder (SP), ISO standard sand, nano-silica (NS) with 300 m2/g specific surface area and 7- 40 nm particle size, polycarboxylate superplasticizer (PCE) and self-prepared nano-C-S-H (NC). The particle size distributions of cement and SP are shown in Figure 1. The chemical compositions of the cement and SP are shown in Table 1. XRD pattern and TEM image of NS are shown in Figure 2.
2.2 Preparation of NC
In the experiment, calcium silicate solution and anhydrous calcium chloride solution were used to prepare NC, and the water-solid ratio of the reaction solution was controlled to be 10 and the calcium-silicon ratio was 1.2. During the process, pH was adjusted to 12 using Na(OH)2 solution. After heating in a water bath at 60 °C for 7 days, the crystals were collected by washing and vacuum filtering, and the resulting solids were ground to obtain NC.
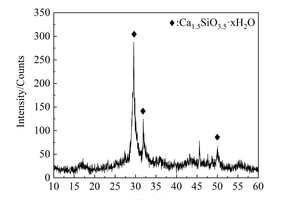
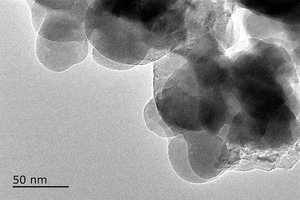
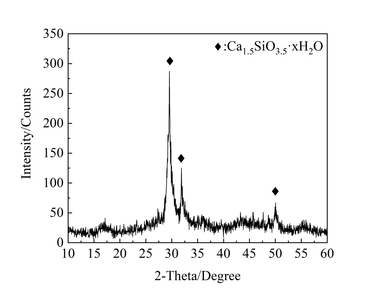
The XRD pattern and TEM image of NC are shown in Figure 3. XRD structural analysis shows that NC is semi-crystallized (Ca1.5SiO3.5·H2O) at 29.55°, 31.88° and 50.05°. TEM analysis indicates that NC was powered by the agglomeration of 40-50 nm spherical particles, and the surface was layered with loose and porous.
2.3 Mix proportion & specimen preparation
The mix ratios of the mortars are shown in Table 2.
The mortar specimens were prepared according to GB/T 17671-1999 [15], the sand-cement ratio was 1:3, and the water-cement ratio was 0.5. SP was used as mineral admixture, replacing cement with 30% equal amount. Nanoparticles were added in an amount of 1%~4% of the quality of binders (The masses sum of cement and slag powder). PCE was added to ensure a similar fluidity level ((185±5) mm) with the same water/binder ratio. To ensure the uniform dispersion of nanoparticles in the paste, nanoparticles were added into water and ultrasonically dispersed for 10~15 min before the test started. The same water-cement ratios as that of mortars were used for the pastes, and the mixing method was referred to GB/T1346-2011 [16].
2.4 Method
The fluidity of cement paste was obtained according to GB/T 8077-2012 [17]. The compressive strength of mortars at 1 d, 3 d, 7 d and 28 d were measured by YAW-300 automatic pressure testing machine according to GB/T 17671-1999 [15]. The setting time was determined according to GB/T 1346-2011 [16]. The resistivity was continuously monitored for 72 h by CCR-3 electrode-free resistivity tester. The phase analysis was performed by Bruker D8 Advance X-ray diffractometer (XRD). DSC-TG analysis was carried out with a STA449C/3/G simultaneous thermal analyzer. The surface morphology of cement paste was observed by a Japanese JSM-6610 SEM in a vacuum environment.
3 Result and discussion
3.1 Fluidity
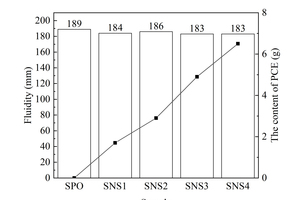
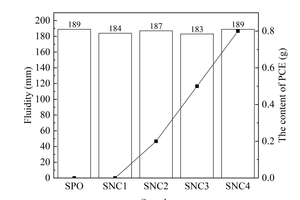
The fluidities of cement pastes of different samples are shown in Figure 4.
As can be seen from Figure 4, at the same water-binder ratio, when the fluidity of cement paste is controlled at a certain level, the content of PCE added to paste increases with the dosage of the two nanoparticles, and the content of PCE required by the sample with NS is much higher than that required by the sample with NC. These indicate that the incorporation of both nanoparticles increases the water requirement of the pastes, and the water requirement of the sample with NS is larger than that of the sample with NC. On the one hand, the specific surface area and the activity of NS are higher than those of NC, and NS could react quickly to form flocculated precipitation and consume free water in the paste after incorporation; on the other hand, agglomeration easily occurs between NS particles due to their large specific surface area and high surface atom energy, and the NS agglomerates cannot be used as fillers to fill the gaps between cement grains and replace the filling water between cement particles [18]. With the combined effects of these two aspects, the water requirement of the NS sample is larger than that of the NC sample.
3.2 Setting time
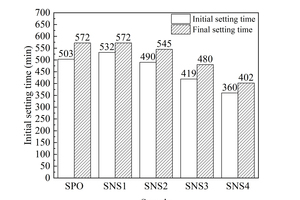
Figure 5 shows the initial and final setting time results of different samples.
As shown in Figure 5, the initial setting time of the paste increases when NS dosage is low (1%). According to relevant studies [19, 20], although the steric hindrance effect of PCE can provide better dispersion, it can also prolong the hydration induction period of the cementitious system and the end time of the induction period is also generally regarded as the initial setting time [21]. Therefore, in the induction period the retarding effect of water reducer is greater than the set-accelerating effect of NS in small dosage. When the NS content is larger than 1%, the setting time is prolonged with increasing dosage. For instance, the initial and final setting time of cement paste are shortened by 143 min and 170 min respectively when the NS dosage is 4%. The addition of NC also reduces the setting time of hydration paste. When the content of NC is 4%, the initial and final setting time are shortened by 103 min and 115 min, respectively. That is, both NS and NC can shorten the setting time, but due to the higher activity of NS, the pro-setting effect of NS is better than NC.
3.3 Compressive strength
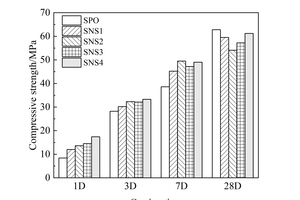
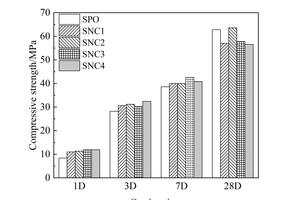
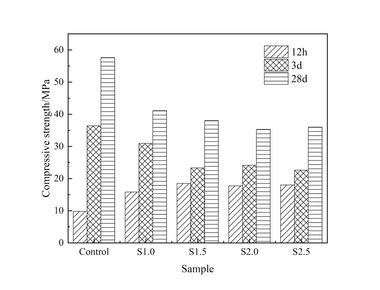
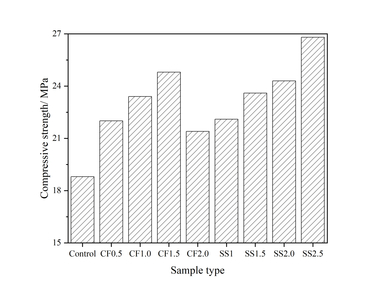
In order to investigate the influence of nanoparticles on the mechanical property of the slag powder/cement system, the compressive strength of hardened mortars with different nanoparticles was tested and the results are shown in Figure 6.
It can be seen from Figure 6 that the early strength of the mortar is improved to some extent by adding nanoparticles. In the dosage range of 1%~4%, adding NS increases the strength by up to 107.14% at 1 d, 18.09% at 3 d and 28.24% at 7 d. Adding NC increased the strength by up to 41.67% at 1 d, 10.64% at 3 d and 11.14% at 7 d, indicating that NS is better than NC in improving the early strength of mortars and the enhancement of matrix strength by the two nanoparticles mainly occurred at early ages, and gradually decreased with time. At 28 d, the strength of mortars with two kinds of nanoparticles appears “delayed development” phenomenon. The reason for this may be that both nanoparticles have good nucleation effect, resulting in too much nucleation in the early stage, and as the hydration time increases more and more C-S-H gels gradually cover the surface of the cement and SP particles, increasing the difficulty of ion migration, and the hydration reaction can only take place in the transition zone of the phase transformation [22].
3.4 Resistivity
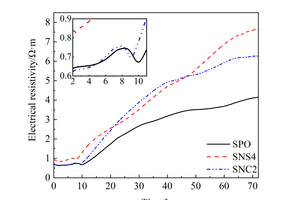
4% NS and 2% NC were selected as the optimal mixing amounts, the resistivity of SPO, SNS4 and SNC2 samples was measured and the results are shown in Figure 7.
As shown in Figure 7, the resistivity of the SNS4 sample was always higher than that of the SPO sample. This is because NS with high pozzolanic activity can rapidly adsorb Ca2+ and OH- in the fluid phase of the paste to generate C-S-H gels [23], which reduces ion concentration and liquid conductivity. On the other hand, the generated C-S-H can be used as crystal nucleus to adsorb Ca2+ and H2SiO42- in the liquid phase to promote crystal growth and fill the liquid phase space, causing the NS4 to have a higher resistivity than that of SPO. When NC was added, an external calcium source was introduced, which increased the ion concentration in the fluid phase of the paste. Therefore, the resistivity up to 5 h is lower than that of SPO. After 5 h, SNC2 sample reaches the saturation ion concentration earlier than the SPO sample, which promotes the nucleation and growth of hydration products and thus limits the liquid conductivity space, and finally the resistivity of the SNC2 sample is higher than that of SPO paste. In other words, the cement hydration can be promoted after the addition of two kinds of nanoparticles. Ultimately, the resistivity relationship after 72 h is SNS4>SNC2>SPO.
3.5 XRD and DSC-TG analysis
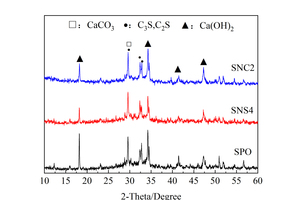
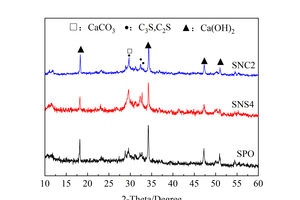
In order to study the influence of two nanoparticles on hydration products of the slag/powder cement system, XRD was used for phase identifications and the results are shown in Figure 8. By comparing the characteristic peaks of each hydration sample in Figure 8, it can be seen that the incorporation of nanoparticles does not cause significant changes in phase composition, and calcium silicate (C3S/C2S), calcium hydroxide (CH) and calcium carbonate (CaCO3) are still the main phases in all those samples.
Figure 8 a shows that the CH and CS diffraction peaks of the SNS4 sample are lower than those of the SPO sample, which is caused by the high surface activity of NS. Specifically, the pozzolanic reaction of NS can consume CH to generate C-S-H gels, which reduces the saturated concentration of Ca2+ in the liquid phase and thus promotes the hydration of cement clinkers. In addition, the CH diffraction peak of the SNC2 sample is lower than that of the SPO sample. The reason for this phenomenon is that the incorporation of NC promotes the dissolution of Ca2+, increases the concentration of Ca2+ in the solution [24], and reduces the nucleation barrier for CH crystal precipitation [25], resulting in the faster generation of C-S-H gels and CH crystals. At the same time, the generated CH stimulates the activity of SP and causes some consumption of CH. When the consumption rate of CH is greater than its formation rate, the diffraction peak of CH would decrease.
Figure 8 b shows that the CH peak of the SNS4 sample is still lower than that of the SPO sample at 28 d, which may be attributed to two factors: Firstly, the addition of NS consumes a certain amount of CH; secondly, NS promotes the hydration of cement to generate more C-S-H gels at early ages, which cover the surface of the cement particles, resulting in the difficulty of ion migration and the slow late hydration rates. At 28 d, the intensity of CH diffraction peak of the SNC2 sample is similar to that of the SPO. This is because although NC can promote hydration of cement clinker and generate more CH crystal, the activation of CH to SP also leads to more CH consumption. Under the combined action of the two, the XRD patterns show comparable CH diffraction peak intensity.
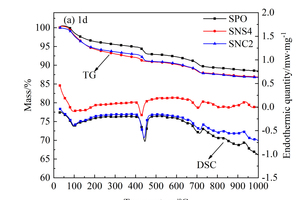
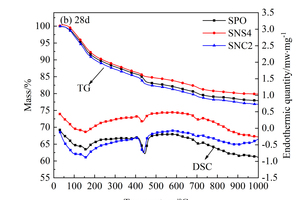
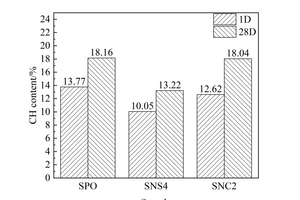
In order to further analyze the influence of the two nanoparticles on hydration products, DSC-TG quantitative analysis was conducted on the above three samples, as shown in Figure 9. From Figure 9, three major endothermic peaks can be found in all DSC curves. The first peak at around 100~200 °C is attributed to the evaporation of the free water and the dehydration of C-S-H gels and ettringite; the second one located at 400~500 °C is caused by the dehydration of CH; the third one at around 700 °C results from the decomposition of CaCO3. Considering CaCO3 as CH carbonization products in the process of preparing the samples, the content of CH can be quantitatively calculated by Formula (1) [26, 27], and the results are shown in Figure 10.
⇥(1)
where WLCH and WLCC represent the mass loss in percentage caused by the decomposition of CH and CaCO3, respectively.
As shown in Figure 10, the contents of CH at 1 d and 28 d in the SNS4 sample are 27.02% and 27.20% lower than that in the SPO sample, respectively. On one side, at early ages the pozzolanic activity of NS allows it to react with CH; on the other side, SP releases silicon phase in the vitreous under alkaline environment with hydration and CH can be consumed in the reaction process with silicon. Therefore, the contents of CH decreased whether at 1 d or 28 d. Moreover, the contents of CH at 1 d and 28 d are also decreased after the addition of NC, but the decrease range is not significant. This is because although the accelerated effect of NC on cement hydration can lead to more CH crystal formation, the activation of CH on SP also leads to more CH consumption. When the amount of CH generation and consumption are close, the content of CH is similar. However, the amount of C-S-H gels generated in SNC2 sample is also more than those in SPO sample, so the strength is slightly higher.
Based on the above analysis, it can be seen that the incorporation of NS would lead to a significant decrease in the content of CH, while the addition of NC has little effect on the content of CH, which is due to the different mechanisms of the two. Specifically, NS can react with CH to generate C-S-H gels, which can be used as new nucleation sites to promote the cement hydration, and NC can be directly used as the crystal nucleus to reduce the nucleation barrier of hydration products, promoting cement hydration to generate more C-S-H gels and CH, and CH can stimulate the activity of SP to further promote the hydration of the system, and thus increase the development of matrix strength.
3.6 SEM analysis
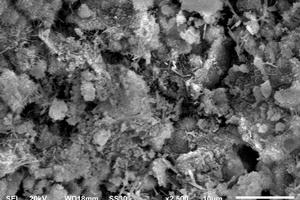
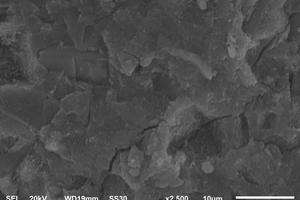
Figure 11 shows the surface morphology of different hydrated samples.
It can be seen from Figure 11 a-c that the hydration degree of samples at 1 d is relatively low, and the hydration products are mainly ettringite, CH and C-S-H gels. As shown in Figure 11 a, the hydration products of SPO are small in quantity and a small amount of ettringite appears. Although the hydration products are connected in clusters, there are also many pores. However, the early hydration degree of the SNS4 sample is higher than that of the SPO sample, and there are more clusters of hydration products in the SNS4 sample than are present in the SPO sample. Therefore, the porosity of the SNS4 sample is also reduced compared with that of the SPO sample. These results indicate that the addition of NS can promote the hydration, accelerate the overlapping and bonding of clusters of hydration products, and thus promote the development of cement strength. The hydration degree of SNC2 is also higher than that of SPO, and the clusters of hydration products are significantly more numerous than they are in the SPO and the SNS4, but the overlap between hydration products is not as tight as that of the SNS4. These results show that the addition of NS and NC contributes to the hydration of the system and the formation of hydration products, and can promote the filling of fine pores. However, at early age, the improvement effect of NS on hydration and the filling performance of fine pores are better than that of NC, which is consistent with the results of setting time and early strength of the matrix.
From Figure 11 d-e, it can be seen that the degree of hydration at 28 d is significantly higher than that at 1 d, and the differences among SPO, SN4 and SNC2 are no longer obvious, which is mainly because the cement clinker and slag powder are constantly hydrated with the extension of age, and the hydration products increase obviously.
4 Conclusion
The addition of NS and NC would increase the water demand and shorten the setting time of the slag powder/cement system. Due to the large specific surface area and high surface activity of NS, the content of water-reducing agent required by the sample mixed with NS is higher than that mixed with NC when the flow degree is controlled uniformly, and the coagulation-promoting effect of NS is better than that of NC.
The incorporation of NS and NC can significantly improve the early strength of the slag powder /cement system. Within the content range of 1% to 4%, NS and NC can increase the 1 d strength by up to 107.14% and 41.67%, respectively, and the enhancing effect of NS on 3 d and 7 d strength is also higher than that of NC.
NS with high activity can quickly absorb Ca2+ and OH- ions in the liquid phase after being mixed into cement-based materials, forming C-S-H gels and reducing the liquid ion concentration, so that the resistivity of SNS4 is always higher than that of SPO. When incorporating NC, the ion concentrations in the liquid phase of the cement paste increase, so the resistivity of SNC2 is smaller than that of SPO up to 5 h. After 5 h, the SNC2 sample reaches the saturation ion concentration earlier than the SPO sample, which promotes the nucleation and growth of hydration products and thus limits the liquid conductivity space, and finally the resistivity of the SNC2 sample is higher than that of the SPO paste. After hydration for 72 h, the resistivity relationship is SNS4>SNC2>SPO.
Both NS and NC can promote the early hydration, decrease the CH content and improve the compactness of the slag powder/cement system, and thus increase the early compressive strength. Simultaneously, because the activity of NS is higher than that of NC, the improvement effect of NS on the early performance of cement-based materials is better than that of NC.
5 Acknowledgement
Financial supports from the National Natural Science Foundation of China (51908434), the Science and Technology Research Program of Hubei Province Education Department (Q20191706) and the Natural Science Foundation of Hubei Province (2020CFB799) are gratefully acknowledged.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.