Accelerating cement hydration with C-S-H seeds
The acceleration of cement hydration through synthetic C-S-H shows great potential in increasing the early strength of cementitious systems. The selection of a suitable calcium-to-silicon ratio and optimized synthesis conditions results in a significant strength gain compared to non-optimized systems. Polymers may contribute during synthesis to stabilizing the C-S-H aggregates, thus enhancing the storage stability of the suspensions.
1 Introduction
According to the European climate and energy policy, the emission of greenhouse gases is to be reduced by 40 % by 2030 compared to 1990 [1]. The German cement industry contributes essentially to the issue with annual emissions of 17450 million t CO2 (2015). Nevertheless, their 1995 self-imposed climate goal of a 28 % reduction of energy conditioned CO2 emissions was achieved in 2012 [2]. The application of alternative fuels, together with more effective kilns and improved clinker mineralogy contributed to the achievement of climate goals just as well as the development of...
1 Introduction
According to the European climate and energy policy, the emission of greenhouse gases is to be reduced by 40 % by 2030 compared to 1990 [1]. The German cement industry contributes essentially to the issue with annual emissions of 17450 million t CO2 (2015). Nevertheless, their 1995 self-imposed climate goal of a 28 % reduction of energy conditioned CO2 emissions was achieved in 2012 [2]. The application of alternative fuels, together with more effective kilns and improved clinker mineralogy contributed to the achievement of climate goals just as well as the development of so-called “low-CO2” cements [2-5]. Another method with which to avoid greenhouse gas emissions is to reduce the clinker share in cements and concretes. The share of CEM II and CEM III cements increased by 28 % between 2004 and 2015 [2]. The reduced clinker share, nevertheless, results in reduced hydration activity, hence excluding these cements from applications that require fast hardening [6, 7].
In addition to ecological considerations, the acceleration of setting and hardening is a practical requirement for applications like repair mortars and 3D printing, and for the precast concrete industry, it becomes a question of productivity, since the time required for early strength development determines the turnover speed of the molds.
Common accelerators for cementitious systems are often accompanied by negative side effects such as enhanced corrosivity or lower long-term strength [8].
The acceleration of cement hydration with nanoparticles, on the other hand, is accompanied neither by higher risk of corrosion nor reduced long-term strength. While unreactive nanoparticles like titanium and zirconium dioxide accelerate by their physical presence alone, the application of pozzolanic nanoparticles leads to additional C-S-H formation and, hence, to enhanced compressive strength [9].
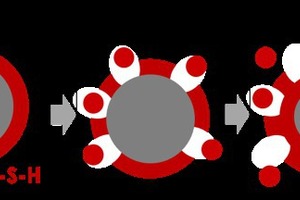
The acceleration of cement hydration with synthetic calcium silicate hydrate nanoparticles is a special case, since C-S-H is the main hydration product of cement. It is assumed that C-S-H particles act as nucleation templates and drive the location of product formation from the clinker surfaces to the pore space. Scanning electron microscope images support this theory by showing a reduced layer of hydration products around the clinker and a denser microstructure in the pore space [10-13].
The current work summarizes the effects of C-S-H seeding based on own investigations and literature-known results. Furthermore, an outlook towards future research fields regarding C-S-H seeding is given.
2 Synthesis of calcium silicate hydrate
As a measure of the efficiency of C-S-H seeds, either the position of maximum heat development or that of compressive strength is determined. The one-day strength gain reported in the literature varies between 4 and 270 % [6, 13-20]. It is obvious that, beyond the dosage, the synthesis method of C-S-H expresses strong influence on their performance. The most basic method for preparing calcium silicate hydrates is via the pozzolanic reaction of calcium hydroxide with silicon dioxide in water. The reaction can be accelerated by mechanical milling so that, besides colloidal silica, less reactive silicate sources like fine quartz also become accessible (Figure 2) [19, 21].
Another method is sol-gel synthesis from organic precursors. For that, calcium ethylate is prepared from metallic calcium and ethanol, and tetra ethylene ortho silicate (TEOS) is added as a silicon source. Hydrolysis is induced by the addition of water, and condensation is completed within several hours (1) to (4). Remnant starting materials are removed by washing with water and ethanol [19, 22].
Hydrolysis:
⇥(1)
Condensation:
⇥(2)
Condensation:
⇥(3)
Incorporation of calcium:
⇥ (4)
Calcium silicate hydrates prepared by the sol-gel method show a distinct morphology and crystallinity compared to pozzolanic C-S-H (see Chapter 3).
A third synthesis method is the co-precipitation of water-soluble calcium salts like CaCl2 or Ca(NO3)2 and alkali silicate (mainly sodium silicate). This method additionally allows the facile preparation of polymer/C-S-H composites [23, 24]. Tuning material properties through polymers is a technique inspired by nature, where numerous examples of xenomorph mineral morphologies and structures with exceptional material properties like sea urchin spines or mollusc shells can be found. The structures are created via non-classical crystallization pathways though the stabilization of mesocrystals by bio-polymers [25-27]. The stabilization of aggregates is essential for the preparation of nanoparticles with defined shapes and sizes (see Chapter 3).
While the earlier described synthesis methods can produce semi-crystalline C-S-H, crystalline calcium silicate hydrates with a defined stoichiometry and morphology are only accessible through energy demanding hydrothermal processes. So far only the impact of afwillite onto the hydration of alite was investigated [28, 29].
3 Properties of C-S-H
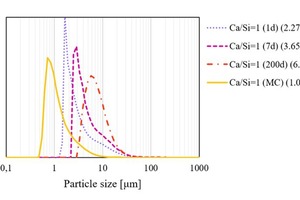
A strongly agglomerated C-S-H gel is formed by the pozzolanic reaction with or without mechanical treatment (Figure 4, right). The size of the agglomerates prepared without mechanical treatment at room temperature (RT) depends on the reaction time (Figure 3), but the aggregates can be broken down to the size of freshly prepared C-S-H (1d) in the case of seven-day-old C-S-H.
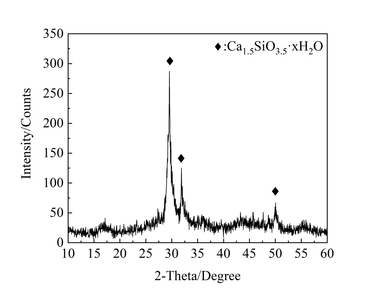
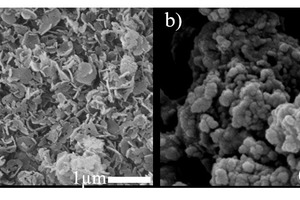
In X-ray diffraction, the pozzolanic C-S-H shows the typical reflexes of C-S-H I. Depending on the calcium-to-silicon ratio (Ca/Si), the position of the basal peak and the intensity of the reflex at 17 °2Θ, which is correlated to the Q2 linked silicate units, both vary. The reduction in basal spacing correlates with BET investigations showing that low-calcium C-S-H has a larger specific surface area than high-calcium C-S-H. 29Si-MAS-NMR measurements demonstrate that the mean chain length rises with the silicon content. For Ca/Si = 1.2, it is only slightly above 2, while for Ca/Si = 0.8 a silicate chain length of approximately 9 is reached. Samples prepared by sol-gel synthesis are X-ray amorphous and show a hump around 20-35 °2Θ. No reflexes matching calcium hydroxide or carbonate were found. SEM images show a platelet-like structure (Figure 4, left).
Organic molecules allow the preparation of numerous xenomorphic C-S-H morphologies: Moghaddam et al. prepared cubic and dendritic C-S-H with calcite nuclei, the morphology of which was controlled by cetyl trimethylammonium bromide. Phattharachindanuwong et al. prepared hollow C-S-H spheres with latex templates, and Plank et al. were able to control the particle size via the side chain length of methacrylic acid–co–ω –methoxy poly(ethylene glycol) methacrylate ester [31-33]. The influence of the organic molecule can be distinguished into two groups: stabilization of aggregates through electrostatic interactions and hydrogen bridges (PVP1-co-PAA2, PAAm3-PAA, peptides) [34, 35], and intercalation into the C-S-H structure (PEG4, HDTMA5 [37, 38]. Anionic and cationic surfactants may act as templates for the growth of xenomorphic morphologies (CTAB6, SDS7, TDAB8, CPB9) [31, 36].
Crystalline C-S-H is characterized by its defined stoichiometry and morphology. Needles, platelets and cuboid structures have been reported (Figure 5) [39].
4 Influence of C-S-H on cement hydration
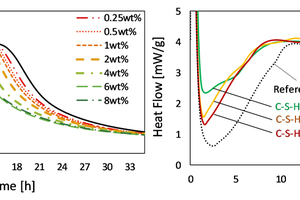
The addition of dispersed C-S-H nanosized particles to cement pastes results in an accelerated hydration rate, while the induction and dormant periods are shortened. The maximum heat evolution shifts to earlier times and higher values, and the time to reach maximum can be reduced to 1/10th of the original time with up to 120 % higher maxima. Nevertheless, an upper limit for the beneficial addition of C-S-H appears to exist, as the addition of higher C-S-H concentration did not further accelerate the hydration (Figure 6, left) [40, 41].
The calcium-to-silicon ratio is one factor influencing the performance of C-S-H seeds. It defines many properties of calcium silicate hydrate (see Chapter 3). Contradictory results regarding the effect of the calcium-to-silicon ratio on the performance of accelerators were published by Land et al. and Alizadeh et al.. The latter found that low-calcium C-S-H were more effective than high-calcium C-S-H [42, 43]. Land et al. found the opposite, but their investigations included C-S-H with a Ca/Si of 2, which is higher than the limit for the preparation of pure C-S-H under the given conditions. For Ca/Si = 2, calcium hydroxide exists as a by-product overestimating the effect of C-S-H alone [43].
Own investigations lend support to the results of Alizadeh et al.. Cement accelerated with 2 wt.- % C-S-H shows an elimination of the dormant period that was more pronounced with low-calcium C-S-H (Figure 6, right).
The performance of the seeds appears to be dependent on the cement mineralogy, as well. The amount of sulphate agents was evaluated as a major parameter; too low sulphate or alkali contents resulted in an inhibition of lower C-S-H concentrations; only the addition of higher quantities resulted in an acceleration. The upper limit of addition was reached later, when the amount of sulphate agents was low [40]. It is assumed that the calcium ion concentration is the decisive factor for this effect, since the sulphate agents contribute to a constant calcium ion concentration in the pore solution, and a reduction in Ca2+ results in acceleration [44]. Besides cement mineralogy, the effect of fineness was also investigated. It was found that finer cements require more C-S-H for the same acceleration, which highlights the importance of C3S dissolution for understanding cement hydration [40].
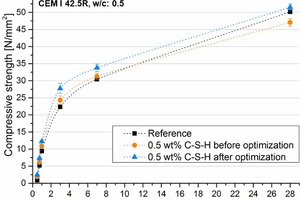
Compressive strength and ultrasonic velocity measurements show results correlating to calorimetry, stiffening and hardening, which are significantly shifted to earlier times by the addition of C-S-H seeds. Additionally, a great potential for optimization was found (Figures 6 and 7) [19, 41].
Kanchanason et al. demonstrated in 2015 that the effectiveness of C-S-H can be enhanced by the formation of polymer composites, i.e., the compressive strength could be improved by 54 % compared to polymer-free C-S-H seeded samples. The size of the resultant agglomerates hereby was found to be the decisive factor [17].
5 The mechanism of action
In cements seeded with C-S-H, the formation of hydration products was observed to have shifted from the clinker particle surfaces to the pore solution. This is attributed to the nucleating effect of the synthetic C-S-H, which acts as a template for the nucleation and growth of hydration products (Figure 8) [6, 20, 40]. Whether or not the change in nucleation location has any impact on the composition of the pore solution is a matter of controversy, since only minor amounts of C-S-H induce a significant acceleration. The reduced layer of hydration products around the clinker may enhance the C3S dissolution [40, 42].
Based on the performance differences of C-S-H with varying Ca/Si, another aspect comes into focus. Since the increased BET surface in low-calcium C-S-H correlates with the increased interlayer spacing, it is assumed that the extra surface is not completely available for product growth but formed internally to a large extent. The fact that the Ca/Si does not alter the heat development after the dormant period, as observed for different C-S-H concentrations, supports this assumption (Figure 6). An explanation for the impact of Ca/Si could be that low-calcium C-S-H is a more favourable template for the nucleation and/or growth of hydration products. Another possibility is the local change in pore solution concentration induced by the calcium uptake of low-Ca/Si C-S-H; the increased interlayer spacing supports ion migration into the structure of calcium silicate hydrate. Further investigations of the pore solution are necessary to lend support to this theory.
6 Summary
The acceleration of cement hydration through synthetic C-S-H shows great potential for increasing the early strength of cementitious systems. The selection of a suitable calcium-to-silicon ratio and optimized synthesis conditions result in a significant strength gain compared to non-optimized systems. Polymers may contribute during synthesis to stabilizing the C-S-H aggregates and enhancing the storage stability of the suspensions. Compared to conventional accelerators, C-S-H was not observed to be corrosive, nor did it compromise the long-term strength when used in suitable concentrations.
Through the application of non-toxic calcium silicate hydrate as aqueous suspension, a very positive benefit-risk ratio of the accelerator was confirmed [45].
A final advantage of C-S-H seeding is that effective seeds can be prepared from cheap starting materials like lime and silica in a facile pozzolanic synthesis, as confirmed by Land et al. [41].
Acknowledgement
The authors would like to thank the Deutsche Forschungsgemeinschaft, DFG, project number STE 1086/15-1, for supporting this work.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.

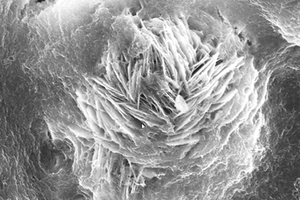
![4 SEM images of crystalline C-S-H: A – killalaite (Ca6.4 [H0.6Si2O7]2 (OH)2), B – gyrolite (Ca1Si24O60 (OH)8(14+x) H2O) in a tobermorite matrix, C – hillebrandite (Ca2SiO3 (OH)2), D – xonotlite (Ca6 [Si6O17] (OH)2). Product formation is dependent on Ca/Si, treatment temperature and duration](https://www.zkg-online.info/imgs/1/4/2/2/0/5/0/tok_f42379da46f896ed83b8c05bcc6691c3/w300_h200_x600_y117_Materials_John_Stephan_Figure4-98314817eb8a190a.jpeg)
![6 Compressive strength development during the first hours of cement hydration and over 28 days with 0.5 wt.-% C-S-H before and after optimization of the synthesis [19]](https://www.zkg-online.info/imgs/1/4/2/2/0/5/0/tok_6079f1232c6ff9dee875360fc5ab7900/w300_h200_x325_y255_Materials_John_Stephan_Figure6a-ec439ee0ad2ec3bb.jpeg)
![7 Ultrasonic sound speed measurement of cement pastes with 0.5 wt.-% C-S-H before and after optimization of the synthesis [19]. The setting time could be accelerated by approximately 1.5 hours through non-optimized C-S-H and by further 2 hours through optimization](https://www.zkg-online.info/imgs/1/4/2/2/0/5/0/tok_520ff251aa0702afeff084d3ef2ed51f/w300_h200_x348_y261_Materials_John_Stephan_Figure7-5f3f2cecac74bd5f.jpeg)