Investigations into the influence
of chemical activators and templates
on cement hydration
The strength of hydrated cement and its cohesive properties are determined to a great extent by the structure of the calcium silicate hydrate phases. There is a description of investigations into the crystallization of calcium silicate hydrate (tobermorite) in the presence of various amino acids that lead to significant changes in the elementary cells.
1 Introduction
The most successful ways of reducing the emission of CO2 during the production of cement are currently based on the use of cement replacement materials and their activation. The investigations and applications have focused on slags, natural pozzolans (trass) and synthetic pozzolans (fly ash, metakaolins and calcined clays) as the cement replacement materials. Low early strengths are often a reason why cement replacement materials are not yet widely used. Familiar options for raising the early strengths and for activation are based on reactions in the early phase of cement...
1 Introduction
The most successful ways of reducing the emission of CO2 during the production of cement are currently based on the use of cement replacement materials and their activation. The investigations and applications have focused on slags, natural pozzolans (trass) and synthetic pozzolans (fly ash, metakaolins and calcined clays) as the cement replacement materials. Low early strengths are often a reason why cement replacement materials are not yet widely used. Familiar options for raising the early strengths and for activation are based on reactions in the early phase of cement hydration.
Possible ways of activating pozzolanic reactions include addition of soluble calcium compounds, raising the pH value and the use of soluble silicates and crystallization nuclei. Increased ettringite formation, and therefore improved early strengths, are often achieved in construction chemistry products by the addition of soluble sulfates and aluminates (Figure 1).
Nanocrystalline calcium silicate hydrates (C-S-H) and crystalline portlandite are formed during the hydration of the main mineral phases of Portland cement. Calcium silicate hydrates (C-S-H) are responsible for the strength development of the hydrated cement and determine its cohesive properties. The exact structure of the C-S-H phases at the nano and micro levels is being intensively investigated because accurate knowledge facilitates the development of optimized structures with significantly improved property profiles [1, 2, 3].
The C-S-H phases consist mainly of tobermorite and jennite structures with a multi-scale porosity and have the appearance of a branched three-dimensional C-S-H nano structure [4, 5]. Investigations show that the addition of nanosilica produces a significant improvement in the performance of cement-based materials [6, 7]. This is attributed to accelerated solvation of the tricalcium silicate, accompanied by more rapid formation of C-S-H [6]. The pozzolanic reaction of nanoscale silicon dioxide with dissolved calcium ions also increases the formation of C-S-H [8, 9]. It is also reported that a reduction of the Ca/Si ratio improves the strength of the cement hydrate. [9, 10].
Microstructural investigations by solid body 29Si NMR have shown that Portland cement containing nano silicon dioxide has a stronger and more stable bonding skeleton because of the formation of denser microstructures through the growth of longer silicon oxide chains [11, 12, 13, 14]. In contrast to naturally occurring crystalline calcium silicate hydrates the C-S-H phases that occur in cementitious systems exhibit only a low structural long-range order.
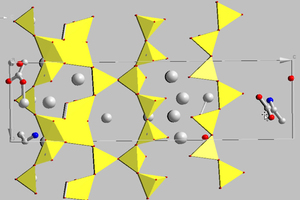
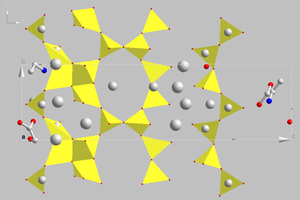
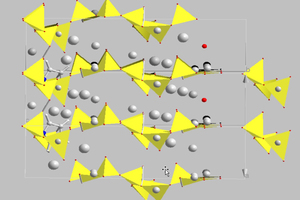
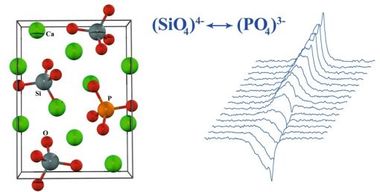
Cementitious C-S-H phases can be modelled using the main constituents, namely tobermorite and jennite. It should be borne in mind that tobermorite occurs naturally in various modifications and these are differentiated essentially by the layer distances between the silicate chains. According to Merlino and Bonaccorsi the characteristic structural element of these minerals is a double layer of Ca-O polyhedra, which are surrounded by linear silicate chains [16]. These polyhedra are linked by “triplet chain” structural units that consist of two Si-O tetrahedra arranged in pairs and joined to one another by bridging tetrahedra of opposing orientation (Figure 2) [15]. Of the phases resembling tobermorite the 11 Å tobermorite structure is highly relevant for describing the real nanocrystalline C-S-H phases. In this monoclinic type of structure the silicate chains condense to form double chains and as a result exhibit voids in the intermediate layers in which water molecules can become embedded. Coordination of Ca2+ ions to the water molecules is also possible. This structure has a C/S ratio of 0.75 with the idealized structural formula Ca4.5Si6O16(OH)·5H2O [15].
In addition to tobermorite, jennite represents another rare but naturally occurring C-S-H phase. The triclinic jennite structure consists, as does tobermorite, of “triplet chain” silicate chains, which, however, are condensed on Ca-O octahedra that are linked at the corners. The structure model gives a structural formula of Ca9Si6O18(OH)6·8H2O with a C/S ratio of 1.5. When compared to the minerals listed here, the C-S-H phases that arise in cementitious systems have a substantially lower long-range order that may be due to the C/S ratios used in the cement mix and the hydration conditions in the cementitious system.
The groups C-S-H (I) and C-S-H (II), which have strong structural and morphological similarities to the hydration products of the pure clinker phase C3S, are used to describe these calcium silicate hydrate phases.
Low-calcium C-S-H (I) phases have a C/S ratio in the range 0.8...1.5 and have a leaf-like form. In contrast, the calcium-rich C-S-H (II) phases can be described by a C/S ratio of 1.4...2.0 and are characterized by a fibrous structure [14]. According to Taylor, C-S-H (I) represents a defective tobermorite structure and C-S-H (II) a defective jennite structure, in which the structure defects result predominantly from defective SiO2 tetrahedra, variable Ca2+ ion concentrations in the intermediate layers or incorporation of foreign atoms [10].
Acceleration or activation by the addition of alkali and alkali-earth ions has been known for a long time [10]. Several variants that influence the rate of solvation of Ca ions in different ways or interfere in the C-S-H gel formation mechanism are also discussed here as reaction mechanisms [17, 18, 19, 20]. Influencing the adsorption on surfaces, chelate formation, influencing the nucleation and changes in the microstructure of the C-S-H phases are discussed as possible mechanisms in this context. A large number of subgroup ions can also accelerate the cement hydration through similar action mechanisms [21].
In the field of biological mineralization processes it is known that crystallization processes are often controlled by templates that, as a rule, consist of special proteins. It is known that the incorporation of organic compounds in the C-S-H structure can also improve the strength and stability, such as, for example, in natural bio-composites [22, 23, 24].
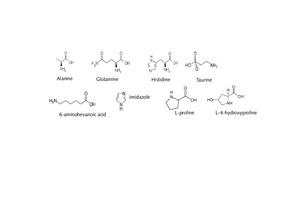
Amino acids, the basic building units of important proteins, were used in this work as functionalizing materials and were tested for their effect with respect to the structure modification of the widespread C-S-H phase tobermorite (Figure 3).
A sol-gel process starting from calcium oxide and silicon dioxide was chosen as the synthesis route for these investigations to avoid the action of disruptive ions.
2 Test procedure
2.1 Starting materials
The pozzolanic synthesis of tobermorite was carried out by mixing commercially obtainable CaO (Alfa Aesar, 95.95 %) with pyrogenic silica (200 m2/g, Evonik) in an aqueous medium with a defined molar Ca/Si ratio. Before they were used the additives were dissolved in double-distilled water. All the samples were stored at a constant temperature of 22.5º C. After reaching the reaction time the samples were washed and dried for 48 hours in a freeze dryer. They were stored in test tubes with air-tight seals for further use. Each experiment was repeated six time to obtain statistically reliable data.
2.2 Experimental procedure
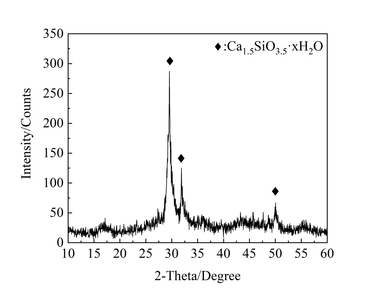
For analysis of the crystal structure by X-ray powder diffractometry (XRPD) the powdered samples were mixed with corundum as an internal standard immediately before the measurement and ground carefully in a mortar. The powder mix was then placed in a sample holder and compacted. A D2-PHASER X-ray diffractometer from Bruker with the Bragg-Brentano geometry was used as the analytical system. The samples were measured with Cu Kα radiation in the 2θ measuring range between 5 º and 60 º with a step width of 0.02 º, for which an 8 s measuring time per 2θ angle and a total measuring time of about 6.5 h (23600 s) were used. Detection was carried out with a one-dimensional Lynxeye band detector.
The chains of the resulting C-S-H phases are only incompletely combined, have defects and can contain foreign ions so they only possess small regions with the degree of order that is necessary for identification by X-ray diffraction [10]. As a result a comparatively long measuring time of 8 s per measuring point was used in order to obtain a sufficient number of pulses in the diffractogram.
The diffraction data were analyzed using Topas 5 (Bruker). Monoclinic 11Å tobermorite (space group: B11m) was used as the structure model for all refinements with the Pawley Fitting. The refinement cycles were repeated until a weighted profile R factor (RWP) of less than 2.5 was obtained.
The structure solution and refinement were carried out with the Endeavour 1.7 g (Crystal Impact) structure solution software using the tobermorite structure database [16] while taking account of the Lennard-Jones potential.
The C/S ratio is crucial for characterizing the C-S-H phases obtained. During the investigations the ratio was determined by atom absorption spectrometry. In each case, determination of the content required a separate, freshly assessed, 8 point calibration with standard solutions. The atom absorption measurements for calcium at a wave length of 422.7 nm and for silicon at a wave length of 251.6 nm were carried out in an AAnalyst 800 spectrophotometer (Perkin-Elmer) using a nitrous oxide/acetylene flame. The sample was digested using lithium metaborate (Alfa-Aesar) in graphite crucibles at 970º C for 15 minutes using a muffle furnace (Heraeus). The solid obtained was dissolved in 4 % nitric acid solution (Carl Roth), diluted to 250 ml and used immediately for determination of the silicon. TOC measurements were carried out in accordance with DIN EN 13137.
3 Results and discussion
The presence of the additives led to sometimes significant changes in the elementary cells. Elementary cells were extended along the c-axis in the presence of the α-amino acids glutamine, alanine and histidine. The greatest effect occurred with glutamine.
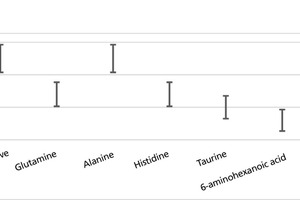
On the other hand, an extension of the elementary cells took place along the a-axis in the presence of imidazole and 6-aminohexanoic acid. A simultaneous change in both the a- and c-axes was detected with taurine (Table 2). The calcium/silicon ratio tends to be reduced by the additives. No significant change in the Ca/Si ratio was determined during the use of alanine (Figure 4).
By determining the levels of TOC in the synthesized products it was possible to calculate the number of amino acids and monomer additives per elementary cell. A ratio of 1 mol alanine per elementary tobermorite cell was determined for alanine. For glutamine, histidine, taurine, 6-aminohexanoic acid and imidazole the ratio in each case was 0.5 mol of the compound per elementary cell.
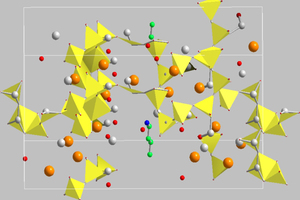
These results served as the basis for carrying out a structure simulation with the aid of the Endeavour 1.7 program from Crystal Impact (Figures 5 and 6).
The structure solution shows that the incorporation of amino acids and monomeric additives takes place preferentially in the region of the interstitial spaces in the double chains.
However, the structure of the amino acids and the monomeric additives affects the precise localization during the incorporation and the accompanying change in the parameters of the elementary cells. The structure of the silicate chains is only slightly distorted by the incorporation of alanine and glutamine but the incorporation of 6-aminohexanoic acid causes a significant change in the basic tobermorite structure.
The modified calcium silicate hydrates were used as crystal nucleation agents in Portland cement to check their influence on the strength formation.
Investigation of the flexural tensile and compressive strengths was carried out on standard mortars after 28 days with a mix formulation containing 50 % CEM I 52,5 R cement (Wittekind Hugo Miebach Söhne KG Portland cement plant), 50 % fly ash (SEAG), 0.1 % nucleation agent w.r.t. cement content and a water/cement ratio of 0.5.
Addition of the tobermorite crystal nucleation agent at the addition rate of 0.1 % relative to the cement content caused no change in the strengths after 28 days. Modification of the tobermorite crystal nucleation agent with glutamine, imidazole or 6-aminohexanoic acid had a negative effect on the strength formation.
However, modification of the tobermorite crystal nucleation agent with alanine led to a slight increase in strength after 28 days.
4 Summary
The strength of hydrated cement and its cohesive properties are determined to a great extent by the structure of the calcium silicate hydrate phases. Investigations into the crystallization of calcium silicate hydrate (tobermorite) in the presence of various amino acids showed significant changes in the elementary cells.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.

![2 Tobermorite structure according to Merlino [16]](https://www.zkg-online.info/imgs/1/4/4/7/3/7/7/tok_6a852cdd10c232a3c41f6b74a71d6253/w300_h200_x545_y393_Materials_Witzleben_Bild_2-43a6d9d3abc36f7c.jpeg)