Effect of sodium sulfate/nano C-S-H
composite on steel slag-cement hydration under steam-curing conditions
In this paper, the influence of sodium sulfate/nano-C-S-H (NC) composite on the compressive strength of a steel slag (30 wt.%)-cement system is explored under the conditions of steam curing, with the influence mechanism being analyzed by XRD, DSC-TG, MIP, SEM and FTIR. Results show that sodium sulfate can significantly improve the early compressive strength of the steel slag-cement system at 12 h, and the best effect is achieved when the dosage is 1.5% (mass fraction), the strength being increased by 88.8%.The incorporation of NC and sodium sulfate together can further increase the compressive strength, and the larger the NC dosage, the more obvious the improvement, mainly because the nucleation effect of NC can improve the hydration degree of pastes and refine the pore structure. However, at 3 d and 28 d, the strength of the single and compound mixing sample is lower than that of the control sample, which indicates that the hydration of cement and steel slag is promoted and the hydration degree of the system is improved at 12 h under steam curing after adding the early strength agent, but it is not conducive to the strength development in late ages. However, the crystal nucleus effect of the compound doped NC increases the nucleation site and promotes the hydration reaction, which makes the strength of the co-doped system still higher than that of the single doped system.
1 Introduction
Steel slag is an industrial waste produced in the process of steelmaking, and its comprehensive utilization rate in China is less than 30% [1]. Steel slag contains C3S and C2S and has cementitious properties, so it can be used as mineral admixture to replace part of the cement in the preparation of concrete, which can not only improve the utilization rate of steel slag and reduce its adverse impact on the environment, but also has great practical significance for the sustainable development strategy. In addition, the incorporation of steel slag is helpful to the formation of...
1 Introduction
Steel slag is an industrial waste produced in the process of steelmaking, and its comprehensive utilization rate in China is less than 30% [1]. Steel slag contains C3S and C2S and has cementitious properties, so it can be used as mineral admixture to replace part of the cement in the preparation of concrete, which can not only improve the utilization rate of steel slag and reduce its adverse impact on the environment, but also has great practical significance for the sustainable development strategy. In addition, the incorporation of steel slag is helpful to the formation of compact structure and can improve the interfacial transition zone of cement-based materials [2]. In China, natural cooling is usually adopted to treat steel slag, so that the content of C3S in the metastable state is less, and simultaneously the steel slag contains more of the RO phase, Fe3O4 and other less active substances [3]. Therefore, adding steel slag would inhibit the early hydration of cement-based materials, resulting in the reduction of early strength, which presents difficulties for meeting the needs of modern rapid construction and limits its application in cement-based materials.
At present, mechanical excitation, chemical excitation and thermodynamic excitation are commonly used to improve the activity of steel slag so as to increase the utilization rate of steel slag in the cement and concrete industry [4]. Steam curing is a kind of thermodynamic excitation, which can improve the early strength of a high volume steel slag-cement system to a certain extent, but still cannot meet the demoulding requirements of the specimen. Sodium sulfate (Na2SO4) has been reported as a kind of ideal hardening accelerator, with the dual excitation effects of alkali and sulfate [5-7]. On the one hand, the addition of Na2SO4 can increase the pH value of liquid phase and create a suitable alkaline environment for the formation of ettringite (AFt). On the other hand, the network structure of vitreous in steel slag is destroyed more thoroughly and decomposed more completely by sodium sulfate, and thus the number of hydration products in the initial stage increases. The active [SiO4]4- and [AlO4]5- dissociated by steel slag can react with Ca(OH)2 to form C-S-H and C-A-H, and C-A-H can react with SO42- ions to form calcium sulfoaluminate hydrate. These hydration products interweave with each other to form a network structure, and simultaneously rapidly fill, block and cut off the capillary pores. Therefore the condensation of cementitious materials is accelerated, the porosity is reduced, and the early strength is improved. Nano-C-S-H (NC) can make the hydration products grow directly on the added crystal nuclei, and reduce the inhibition effect on the dissolution of C3S, which could advance the hydration induction period, shorten the initial and final setting time of hydrated paste, and promote the early hydration and strength development [8, 9]. It can be predicted that under steam curing conditions, the Na2SO4/NC combined used can significantly improve the early strength of the high volume steel slag-cement system.
In this paper, the influence of Na2SO4/NC compound on the strength of a steel slag-cement system under steam curing condition is discussed, and the hydration characteristics of the system are explored by means of XRD, DSC-TG, SEM-EDS, MIP and FTIR, so as to provide a certain theoretical reference for the research and use of high volume steel slag concrete.
2 Experiment
2.1 Raw materials
P·O 42.5 ordinary Portland cement and steel slag (SS) were used in all mixes. The density of steel slag is 3.2 g/cm3, which meets the quality requirements specified in GB/T 20491-2017. Sodium sulfate (Na2SO4) is analytical pure. Nono-C-S-H (NC) is self-made in the laboratory, using sodium silicate solution and anhydrous calcium chloride solution as raw materials, and in the preparation process the water-solid ratio of the solution was controlled to 10 and the mole ratio of calcium to silicon was controlled to 1.2. The main chemical compositions of cement and steel slag powder are shown in Table 1.
2.2 Sample preparation
The mix ratios of the pastes are shown in Table 2.
Pastes were cast with a water-binder ratio of 0.38. The steel slag (SS) content in the sample accounts for 30% of the total cementitious materials. The added dosage of Na2SO4 and NC were related to the amount of cementitious materials, which are 1.0%~2.5% and 1%~4% of the total mass of cement and SS, respectively. The paste samples were prepared in prismatic molds (40 mm × 40 mm × 160 mm), and after curing in a steam curing box at a temperature of 60 °C for 12 h the molds were removed and placed in a standard curing room until the set age, and then the compressive strength test was carried out.
In order to prepare the samples for XRD, DSC-TG, FTIR, SEM and MIP microscopic tests, the small fragments obtained from the middle part of the pastes were immersed into anhydrous ethanol for 3 days, and then dried at 60 °C in a vacuum drying oven. After that, some small pieces were ground into powder for the tests of XRD, DSC-TG and FTIR, and the other small pieces were taken for SEM and MIP tests.
2.3 Test procedure
A YAW-200/300 automatic pressure testing machine was used to test the compressive strength and the test method was carried out according to GB/T17671-2021 [10]. A STA449C Advance X-ray diffractometer (XRD) was used to analyze the phase composition of the samples, a STA449C/3/G synchronous thermal analyzer was used for the DSC-TG analysis, a PoreMaster-33 mercury injection porosimeter (MIP) was used to test the porosity and pore size distribution of the samples, a JSM-6610 electronic scanning electron microscopy (SEM) was used to observe the morphology of the samples and a Nicolet iS50 Fourier transform infrared spectroscopy (FTIR) was used to analyze the chemical structure of the samples.
3 Results
3.1 Compressive strength
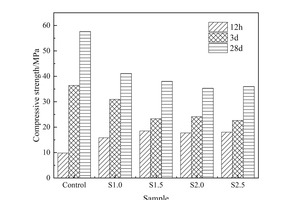
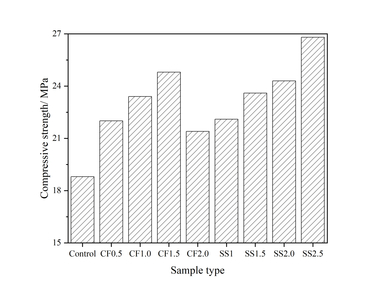
Figure 1 (a) shows the influence of Na2SO4 alone on the compressive strength of a steel slag-cement system. At 12 h, the strength of the test block first increases and then decreases with the increased content of sodium sulfate, the strength reaching its maximum when the Na2SO4 content is 1.5%, which is 88.8% higher than that of the control sample. This is because sodium sulfate, as a strong electrolyte, can provide SO42- and Na+ for the reaction and promote the early hydration of C3S and the generation of hydration products. In addition, CaSO4 and ettringite (AFt) generated during the reaction can fill the pores in the hydration structure and improve the compactness of the hardened pastes [11, 12], thus increasing the strength at 12 h. The strength of S1.5 at 3 d and 28 d was lower than that of the control sample, mainly because the hydration reaction of the paste was too fast when sodium sulfate was added under the condition of steam curing, OH- would combine with Ca2+ and crystallize and enrich in the form of Ca(OH)2, which restricts the continuous hydration and thus affects the reaction degree of the steel slag-cement system in the later stage. Simultaneously, C3S is encapsulated by the hydration products, which makes it difficult to contact with water for a hydration reaction [13, 14], resulting in a later strength reduction.
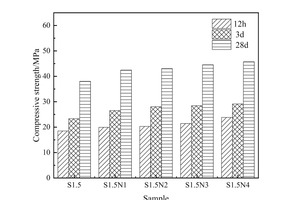
As shown in Figure 1 (b), on the basis of adding 1.5% sodium sulfate the strength of the sample increases with the rise in content of NC, but the promotion effect decreases in line with increasing age. Specifically, the strength of the S1.5N4 sample at 12 h is 28.6% higher than that of the S1.5 sample, but are only 24.9% and 20.3% higher at 3 d and 28 d, respectively. The strength enhancement effect of NC is mainly due to the following reasons: NC can provide crystal nuclei for the hydration of cement-based materials, and hydration products preferentiously grow on the surface of crystal nuclei [15], which significantly reduces the nucleation barrier of hydration products [16] and thus improves the hydration degree and the strength.
3.2 XRD Analysis
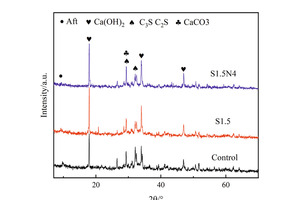
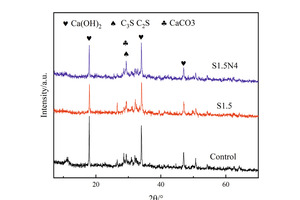
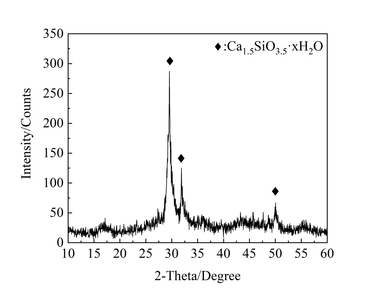
Figure 2 shows the XRD spectra of the control sample, S1.5 sample and S1.5N4 sample at 12 h and 28 d. It can be seen from Figure 2 that the incorporation of sodium sulfate and NC does not change the phase types of a steel slag cement system, which are mainly Ca(OH)2 (CH), unhydrated calcium silicate (CS: C2S/C3S) and calcium carbonate (CaCO3).
Figure 2 (a) shows the XRD spectra of samples at 12 h. Compared with the control sample, the CH content in both the single-doped system and the co-doped system is significantly increased, that of the single-doped system being higher than that of the co-doped system, while the CS contents in the pastes have both decreased. The reason for this phenomenon is that SO42- and Na+ produced by sodium sulfate dissolved in water can react with CH to generate CaSO4·2H2O and NaOH, which reduces the amount of CH [17], while the concentration difference between ions inside and outside the coating layer of C3S increases, accelerating the early hydration of C3S and promoting CH generation. Simultaneously, the alkaline environment promotes the disintegration of the vitreous structure of steel slag, stimulates its activity and improves its hydration degree and CH consumption, thus promoting the hydration of C3S. However in general, the CH production is higher than the consumption [18]; therefore, the addition of Na2SO4 improves the intensity of the CH diffraction peak. The reason why the intensity of the CH diffraction peak decreases in the co-doped system is that nano-C-S-H has a synergistic effect with sodium sulfate. The crystal nucleus effect of NC can make the hydration products grow on its surface, accelerate the hydration process, further improve the alkaline content in the pastes, and stimulate the active ingredient in steel slag to react with CH [19], while the amount of CH consumed is greater than that produced, resulting in a decrease in CH content.
As shown in Figure 2 (b), the CH diffraction peak of the sample mixed with Na2SO4 at 28 d is lower than that of the control sample because the hydration reaction rate of the sample mixed with Na2SO4 is faster in the early stage and the hydration products are wrapped on the surface of unhydrated C3S, which hinders the further hydration reaction [20, 21]. The reason why the intensity of the CH diffraction peak in the co-doped system is still higher than that in the single-doped system is that the hydration products grow on the NC crystal nucleus without dependence on C3S, and the liquid phase Ca2+ concentration is reduced due to the nucleation effect of NC, which continuously promotes the hydration of the sample from 12 h to 28 d, thus increasing the CH content compared with the sample single-doped with Na2SO4.
3.3 DSC-TG analysis
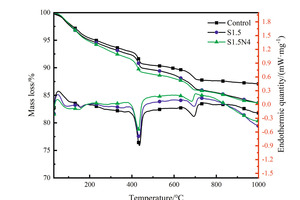
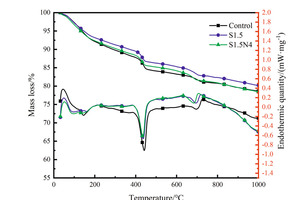
The DSC-TG curves of the hydration sample of steel slag-cement system at 12 h and 28 d are shown in Figure 3.
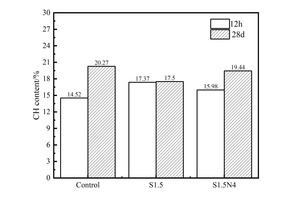
It can be seen from the Figure 3 that there are three stages of mass loss in TG curves. The first endothermic peak around 100-200 °C is related to the evaporation of free water and the dehydration of AFt and C-S-H gels, the second one located at 400-500 °C corresponds to the decomposition of CH crystals and the third one at about 700 °C corresponds to the decomposition of calcium carbonate. According to the research of Zhang et al. [22], the CH content can be calculated, and the results are shown in Figure 4.
As can be seen from Figure 4, at 12 h, the CH content in the control sample is the lowest, followed by the S1.5N4 sample, while the CH content in the S1.5 sample is the highest; However, at 28 d, the CH content of the control sample and the S1.5N4 sample is higher than that of the S1.5 sample. All of these are consistent with the results of XRD.
3.4 SEM analysis
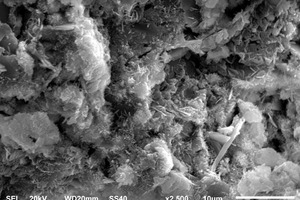
Figure 5 shows the SEM images of different samples hydrated for 12 h and 28 d under steam curing conditions.
From Figure 5 (a)~(c), at 12 h it is obvious that there are less C-S-H gels and AFt in the control sample, and the overall structure is porous and loose. After mixing Na2SO4, the C-S-H gels are significantly increased, which cover the surface of the unhydrated materials and intersperse with the needle-rod AFt to form a whole, decreasing the porosity and increasing the uniformity and density of the overall structure. When NC and Na2SO4 are used in combination, the filling and nucleation effect of NC reduces the pores in the structure and promotes the generation of hydration products, while the C-S-H gels and AFt in the sample are lapped to construct the system structure, further reducing the porosity. As can be seen from Figure 5 (f)~(h), at 28 d the hydration products of the control sample are connected into one piece, showing a tight structure. However, there are more pores in the structure of the S1.5 sample, mainly because the unhydrated C3S is wrapped by the early hydration products, which is not conducive to its later hydration reaction. In addition, compared with the S1.5 sample, the structure of the S1.5N4 sample is more complete, but its compactness is still lower than that of the control sample. All of these are consistent with the strength results.
3.5 FTIR analysis
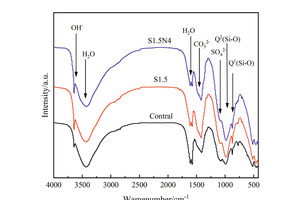
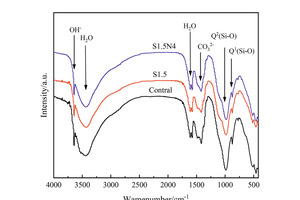
Figure 6 shows the FTIR spectra of the sample at 12 h and 28 d.
As shown in Figure 6, the O-H vibration peak related to the hydration product CH appears around 3643 cm-1, while the H-O-H bond stretching vibration peak and bending vibration peak related to H2O appeared around 3430 cm-1 and 1610 cm-1 respectively. The SO42- vibration peak related to AFt appears around 1100 cm-1, while the Q1 and Q2 vibration peaks related to C-S-H appear around 875 cm-1 and 984 cm-1 respectively. At 12 h, the Q2 vibration peak of the S1.5 sample migrates to a higher frequency (from 983 cm-1 to 985 cm-1) compared with that of the control sample, indicating that Na2SO4 doped alone could increase the degree of polymerization of Si-O tetrahedron[23]. While the Q2 vibration peak of the S1.5N4 sample moves to a lower frequency (985 cm-1 to 984 cm-1) compared with that of the S1.5 sample, and meanwhile, the peak value of the Q2 vibration peak of S1.5 and S1.5N4 samples is higher than that of the control sample, and that of the S1.5N4 is higher than that of the S1.5 sample, indicating that the mixture of NC and sodium sulfate could increase the production of C-S-H gels, and also raise the degree of polymerization and chain length of Si-O tetrahedron to a certain extent.
At 28 d, the intensity of the Si-O vibration peak enhances compared with those at 12 h, indicating that the amount of C-S-H gels increases in line with increasing curing time. In addition, the strong-to-weak sequence of vibration peaks of Q2 is control sample, S1.5N4 sample and S1.5 sample, showing that the amount of C-S-H is the highest in the control sample and the lowest in the S1.5 sample. All of these are consistence with the results of XRD.
3.6 Pore structure analysis
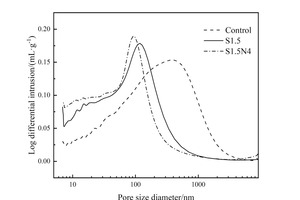
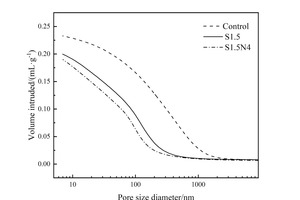
The effects of Na2SO4 and Na2SO4/NC on pore structures of the slag steel-cement system were characterized by MIP, and the pore size distributions of different pastes at 12 h are shown in Figure 7. The research of Wu [24] pointed out that pores in cement matrix composites can be divided into four categories: harmless pores (less than 20 nm), less harmful pores (between 20 nm and 50 nm), harmful pores (between 50 nm and 200 nm), and very harmful pores (bigger than 200 nm). The parameters of the pore structures are listed in Table 3.
As can be seen from Figure 7 and Table 3, the addition of Na2SO4 significantly reduces the total pore volume, the number of very harmful pores and the most probable aperture of the pastes, obviously improving the pore structure, which is mainly caused by the promotion effect of Na2SO4 on the hydration of the steel slag-cement system. Moreover, the addition of Na2SO4/NC can further refine the pore structure of the pastes. Specifically, the total pore volume, the number of very harmful pores and the MPA are all decreased by the incorporation Na2SO4/NC. In addition to the synergistic effect of Na2SO4/NC in further promoting the hydration of the steel slag-cement system, the filling effect of NC is also one of the reasons for the optimization of the pore structure. In general, the reduction of the total pore volume and the refinement of the pore structure can improve the compressive strength, which can be used to explain the change of compressive strength at 12 h.
4 Conclusion
The main conclusions of this study are as follows:
The addition of 1.5% Na2SO4 alone can increase the compressive strength of thesteel slag-cement system at 12 h by 88.8% under steam curing. The incorporation of Na2SO4/NC can further improve the compressive strength of the system at 12 h, and the compressive strength of the sample with 1.5% Na2SO4 and 4% NC is 28.6% higher than that with 1.5% Na2SO4 alone.
At 12 h, the total pore volume, the number of very harmful pores and the most probable aperture of the steel slag-cement system can be reduced by mixing Na2SO4 alone. The addition of Na2SO4/NC can further promote the formation of hydration products, increase the contents of harmless pores and less harmful pores, and further refine the microstructure of the paste.
The compressive strength of the sample with Na2SO4 or Na2SO4/NC is lower than that of the control sample at 3 d and 28 d, and the strength of the co-doped system was still higher than that of the single-doped system.
5 Acknowledgement
Financial supports from the Innovation and Entrepreneurship Training Program for College Students in Wuhan University of Science and Technology (22Z023) and State Key Laboratory of Silicate Materials for Architectures (Wuhan University of Technology) (SYSJJ2022-20) are gratefully acknowledged.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.