Influence of sodium sulfate on hydration and microstructure of cement high volume fly ash system under steam curing
The influence of sodium sulfate (SS) addition on hydration and microstructure development of the cement high volume fly ash (CHVFA) system under steam curing is investigated in this paper. The compressive strength of CHVFA mortars was tested with different SS dosages. Results show that the addition of SS increases the strength obviously when its content is less than 2 %, and that further increase in SS content is not conducive to the development of strength. The mechanism of the positive effect of SS is analyzed by X-ray diffraction (XRD), differential scanning calorimetry-thermogravimetry (DSC-TG), scanning electron microscopy (SEM), mercury intrusion porosimetry (MIP) and nuclear magnetic resonance (NMR). The results reveal that SS incorporation accelerates the hydration of cement and fly ash, decreases the porosity and the content of calcium hydroxide (CH) and increases the polymerization degree of C-S-H, thus enhancing the compressive strength of mortars.
1 Introduction
Coal is the main source of energy in China, and in current and coming decades, it will still play the greatest role in the primary energy structure. Fly ash (FA) is the inevitable product of coal-fired power generation, and every 2 t of coal consumption can produce 1 t of FA. As recently as 2015, FA output in China reached an estimated 620 million t, i.e., the highest level in the world [1]. That is not only a great waste of resources, it also pollutes the environment, vitiates much farmland and affects the sustainable development of the industry when large amounts of solid waste...
1 Introduction
Coal is the main source of energy in China, and in current and coming decades, it will still play the greatest role in the primary energy structure. Fly ash (FA) is the inevitable product of coal-fired power generation, and every 2 t of coal consumption can produce 1 t of FA. As recently as 2015, FA output in China reached an estimated 620 million t, i.e., the highest level in the world [1]. That is not only a great waste of resources, it also pollutes the environment, vitiates much farmland and affects the sustainable development of the industry when large amounts of solid waste are not put to a rational use. Using industrial waste as an admixture in concrete engineering is one major option for achieving highly efficient utilization of FA. It not only reduces the consumption of natural resources, but also offers advantages that other materials cannot substitute, such as meeting the requirements of green concrete. The advantages of FA can be summed up as four key points:
Firstly, FA microspheres are conducive to cement particle dispersion, and they significantly reduce the required content of mixing water while guaranteeing the required workability of concrete [2]
Secondly, the secondary hydration reaction of FA mainly occurs in the pores, which would reduce the porosity of the cement matrix, thus considerably increasing the compactness and durability of cement-based materials [3]
Thirdly, FA, which does not take part in the pozzolanic reaction, can be used as micro-aggregate to fill the pores between aggregate particles, hence further improving the density of concrete [4]
Finally, the hydration heat and the hydration exothermic rate can be markedly reduced by the addition of FA, which also reduces the concrete temperature rise to avoid generating cracks [5-8].
It is well known that improving construction efficiency, shortening the turnaround time and assuring quality are the research emphases of precast concrete, and that the key to solving these problems is to improve the early strength, so that the concrete gains sufficient strength for demoulding as quickly as possible. However, the early strength of cement-based materials containing high volume fly ash (HVFA) develops slowly, a fact that presents numerous difficulties for its practical application in precast concrete. A common way of activating fly ash is steam curing: under high-temperature conditions, the structure of a vitreous network is more easily damaged, and the active Al2O3 and SiO2 of fly ash are more prone to dissolution. This accelerates the transfer rate of the mineral structure and the formation of hydration products. Despite this, when the FA content is higher than 20 %, the compressive strength would decrease rapidly [7, 8]. The addition of exciting agents renders the early age activity of FA conducive to the development of early strength, and much pertinent effort has been invested by many scholars and experts all over the world. Sodium sulfate (Na2SO4) is a kind of ideal excitation agent, since it has the dual excitation effects of alkali and sulfate [9-11]. On the one hand, AlO2- in its liquid phase reacts with Ca2+ and SO42- to generate ettringite (AFt), which increases the dissolution of FA at initial stages and accelerates the pozzolanic reaction between lime and fly ash. Also, the compactness of the AFt layer is lower than that of C-S-H, which is conducive to the diffusion of Ca2+ into the interior of FA particles, where it reacts with the active SiO2 and Al2O3 and makes the activity of FA continue on [9-16]. On the other hand, the addition of Na2SO4 increases the alkalinity of the system, and the weak acidity of chemical composition of FA makes its activity more easily observable in an alkaline environment [9-16]. The study in [9] showed that the addition of Na2SO4 significantly increased both early and later strengths of lime fly ash and that a large amount of AFt is also formed due to the additional SO42-. [17] provided detailed insights into the reaction kinetics, hydration products and fundamental material structure of high volume fly ash-OPC blends activated by a neutral salt such as sodium sulfate, and found that 70 % a reduction in clinker factor results in only a 30 % reduction in strength, due mainly to pore structure refinement and pozzolanic reaction acceleration. [18] investigated the microstructure and strength of a class-F fly ash-based geopolymer containing sodium sulfate as an additive. The authors discovered that the addition of 2 wt% and 4 wt% sodium sulfate produced geopolymers with high strength, and they attributed the increase in strength to an altered pore size distribution in the samples.
Although the effects of Na2SO4 on the early hydration and pozzolanic activity of the fly ash system have been extensively studied, very limited results are available concerning its function on the CHVFA system under steam curing conditions. Thus, in this paper, the effects of Na2SO4 on the hydration and microstructure of an early-age, steam-cured CHVFA system are researched. Moreover, XRD, MIP, DSC-TG, SEM and 29Si NMR are also used to investigate the governing mechanisms behind those effects.
2 Experimental
2.1 Materials
Portland cement (PC) CEM I 42.5 produced by Huarun Cement Co. Ltd is used in all mixes. The secondary fly ash (FA) produced at the Yangluo power plant in Wuhan, China, is used as supplementary cementitious material, in line with national standard GB1596-91 [19]. The analytically pure sodium sulfate (SS) produced by Sinopharm Chemistry Reagent Co., Ltd. is used as a reactive activator for the FA. Liquefied SDS (sodium dodecyl sulfate)-type polycarboxylate superplasticizer with a solid content of 40 % was admixed to reduce the specific volume of water in order to maintain the proper fluidity. Table 1 shows the chemical composition of PC and FA. Table 2 reflects the physical properties of PC.
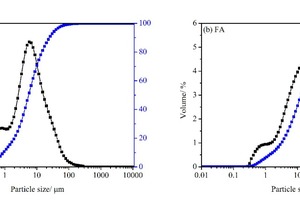
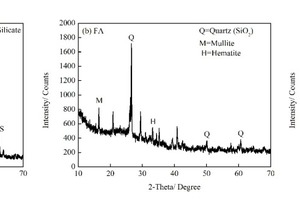
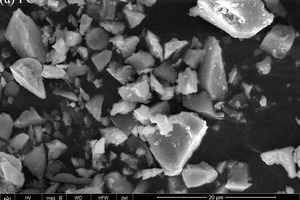
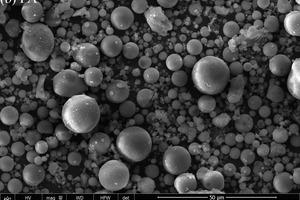
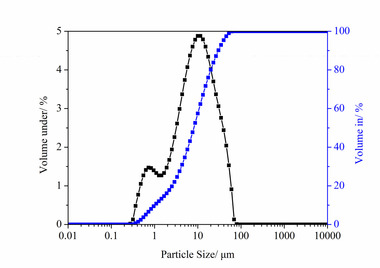
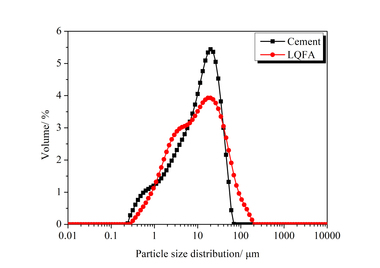
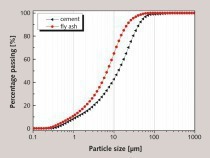
The particle sizes of PC and FA were tested and established using a Mastersizer 2000 laser particle size distribution instrument, as shown in Figure 1. The phase constitutions and microstructures of PC, FA and SS were studied by XRD and SEM, respectively, as shown in Figure 2 and Figure 3. SEM images show that FA particles are perfectly spherical in shape and that the PC particles are somewhat irregular. Standard sand in accordance with GB/T14684-2011 [20] produced in Xia’men, China, was used as aggregate for the mortars. Deionized water was used for all mixtures and experiments.
2.2 Mix proportions and sample preparation
The mix ratios of cement pastes and mortars are shown in Table 3. Cement mortars were cast at a sand-binder ratio of 3 and a water-binder ratio of 0.5. Binder includes PC, FA and SS. SS replacement ratios of 0 %, 1 %, 1.5 %, 2 % and 2.5 % by mass of PC and FA were used for investigating the mechanical properties. The cement mortar samples were prepared according to GB/T17671-1999 [21] in prismatic molds of 40 x 40 x 160 mm and cured in a rapid curing oven at a temperature of 65±2° C and a relative humidity of 95 %~100 % for 12 hours. In order to prepare the samples for SEM, the small fragments obtained from the middle part of mortars at a certain age were put into acetone solution for 3 days and then dried at 60° C for 8 h.
All specimens for XRD, TG-DSC and NMR were molded into squares of cement pastes measuring 40 x 40 x 40 mm. After a certain period of curing as mortars, the hydration of the pastes was stopped by submerging the center part of the crushed samples in acetone solution, after which the samples were oven-dried at 60° C for 8 h and then hand ground in an agate mortar so as to pass the 200 mesh size sieve. The sample for MIP was prepared in the same way as the sample for SEM but by the use of cement paste.
2.3 Test procedure
2.3.1 Mechanical properties
The compressive strength of the mortars was measured according to GB/T 17671-1999 [21] using a WYA-300 series fully automatic test machine with a loading rate of (2400 ± 200) N/s. To achieve reliable results, each test was repeated six times and the data averaged.
2.3.2 Pore structure analysis
The pore structures of the cement pastes were tested using a Quantachrome Autoscan-60 mercury intrusion porosimeter (MIP). The measurable aperture ranged from 7 nm to 200 μm. In the measurement process, the highest pressure was 300 MPa, and the contact angle was set to 130 °.
2.3.3 XRD analysis
A Bruker D8 Advance XRD device with a Cu kα X-ray source at 35 kV and 30 mA was used to determine the phase composition of the samples. During data collection, the step-length was 0.02 °, the scanning rate 8 °/min, and 2- theta ranged from 7 ° to 70 °.
2.3.4 DSC-TG analysis
A Netzsch STA449F3 simultaneous thermal analyzer was used for DSC-TG analysis, which was performed at a heating rate of 10° C/min from 40° C to 1000° C in a nitrogen atmosphere. During the tests, a decalescence peak appearing at about 450° C was caused by the decomposition of calcium hydroxide (CH) into lime, and a peak appearing at about 700° C was caused by the thermal decomposition of CaCO3 [22, 23]. Considering that CaCO3 can be regarded here as the carbonization product of CH, the CH content, CH (%), can be calculated as follows:
⇥(1)
where WLCH and WLCC are percentage weight losses attributable to the decomposition of CH and CaCO3, respectively; MWCH , MWH and MWC are the CH molecular weight, the H2O molecular weight and the CO2 molecular weight, respectively.
2.3.5 29Si MAS-NMR analysis
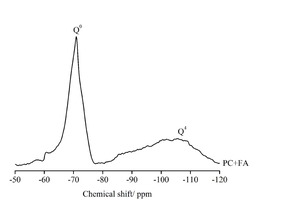
The composition and structure of Si-based substances were qualitatively analyzed by means of a Bruker Avance III 400 Solid-state 29Si magic-angle spinning nuclear magnetic resonance (MAS-NMR) spectrometer operating with a magnetic field at 120 MHz and a rotational frequency of 6000 Hz. A 4 mm zirconia rotor with an accessible volume of 0.115 cm3 was used in all tests. The samples were collected for 2000 scans consisting of a single pulse of width 5 μs followed by a relaxation time of 10 s. As the previous study shows, there are six major forms of peaks in 29Si MAS-NMR spectra of fly ash-cement system, whereas Q0, Q1, Q2, Q3 and Q4 correspond to a silicon-oxygen tetrahedron connected to 0, 1, 2, 3 and 4 silicon-oxygen tetrahedron, respectively, and Q2(Al) represents a silicon-oxygen tetrahedron chain unit connected to one aluminium-oxygen tetrahedron [24, 25].
Q0, which peaks at about -70 ppm, represents unreacted clinker in cement [26]. C-S-H, as the primary hydration product, is identified through resonances at about -79 and -85 ppm, assigned to the species Q1 and Q2, respectively [27]. The aluminum present in the system can modify the C-S-H structure by substitution of tetrahedral Al into the bridging sites to obtain Q2(1Al) sites [24]. Q3 and Q4, which show the 29Si chemical shift peaks at about -89 and -110 ppm, respectively, are the characteristic peaks of unreacted FA [28, 29]. The hydration degree of cement (aOPC), the reaction degree of FA (aFA), the average chain length (ACL) used to describe the polymerization degree of C-S-H, and the Al/Si ratio of C-S-H can be calculated using the following formula [24, 30, 31]:
⇥(2)
⇥(3)
⇥(4)
⇥(5)
where I0(Q0) and I(Q0) represent the integrated intensities of signal Q0 for the cement before and after hydration, respectively; I(Q1), I(Q2) and I[Q2(Al)] represent the integrated intensities of signals Q1, Q2 and Q2(Al), respectively. I0(Q3) and I0(Q4) represent the integrated intensities of signals Q3 and Q4 for FA prior to secondary hydration, respectively.
2.3.6 Morphology and elemental composition
The morphological characteristics of the mortars were determined using a Quanta 200 FEG-SEM set for a 7~15 kV accelerating voltage and a 10 mm working distance in the low vacuum mode. The surface scans of the interfacial transition zone (ITZ) between the aggregate and the cement matrix were analyzed by an SEM attachment–X-ray spectrometer system (energy dispersive spectroscopy, EDS), which were used to measure the elements distribution of ITZ.
3 Results and discussion
3.1 Compressive strength
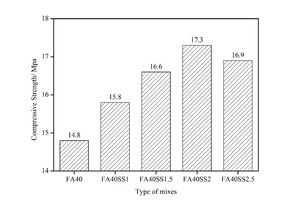
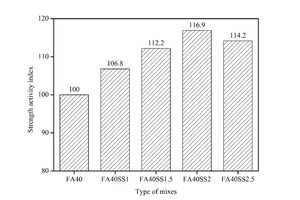
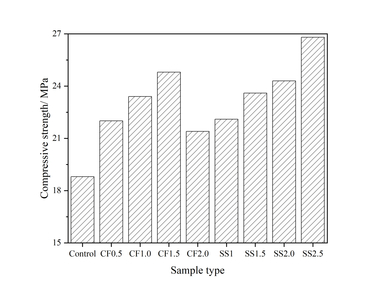
The effect of SS incorporation on the compressive strength of mortars with 40 % FA after 12 hours of steam curing is shown in Figure 4.
As Figure 4 demonstrates, the strength increases with the addition of SS and when the SS content is below 2 %, and the higher the mix ratio, the greater the improvement. For example, 1 %, 1.5 % and 2 % SS increase the strength by 6.8 %, 12.2 % and 16.9 %, respectively (see Figure 5). This is mainly attributed to the promotion effect of SS on the secondary hydration of FA. Specifically, on the one hand, SO42- reacts with active Al2O3 in the liquid phase to form ettringite (AFt) under the action of Ca2+, and the compactness of AFt is less than that of C-S-H, which is conducive to CH diffusing into FA and reacting with active SiO2 and Al2O3 inside sinking and floating beads [9-16]. On the other hand, the addition of Na2SO4 increases the alkalinity of the system, which improves the dissolution of active SiO2 and Al2O3 in the form of SiO44- and AlO2- from the surface of FA, thus promoting the secondary hydration of FA [9-16]. Moreover, the consumption of CH by FA further promotes cement hydration. In addition, the generated CaSO4 and AFt have a certain expansion effect, which can fill some of pores and increase the compactness of the entire system [32]. All of this tends to increase the strength of mortars. However, when the NS content increases to 2.5 %, the strength decreases to a certain extent compared to the sample with 2 % NS. Poor cubage stability caused by the generation of AFt after the cement hardens may play an important role here. According to the provisions of GB175-2007 [33], the content of SO3 in fly ash cement should be less than 3.5 %. The SO3 contents in cement and FA are 2.73 % and 1.59 %, respectively, and calculations show that the dosage of SS should not exceed 2.30 %. So the optimum content of SS is 2 %.
3.2 MIP analysis
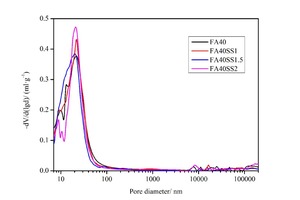
Regarding the effects of porosity on compressive strength, pores in cementitious composites can be divided into four types: harmless pores (smaller than 20 nm), less harmful pores (between 20 and 50 nm), harmful pores (between 50 and 200 nm) and very harmful pores (larger than 200 nm). The results of porosity testing for CHVFA samples with different contents of SS after 12 hours of steam curing are shown in Figure 6 and Table 4.
It is clear that the porosities and total pore volumes decrease noticeably with the addition of SS, and it is easy to see that the pore size distributions are similar for all samples. Thus, it can be concluded that the addition of SS only can change the porosities and total pore volumes but has little to do with the pore size distributions. This is probably caused by the formation of loose AFt. To a certain degree, the changes in compressive strength can also be attributed to the variations in porosity and pore size distribution. There is a rule stating that, the higher the porosity, the larger the pores, and the lower the strength. The strength results discussed in 3.1 are in complete accordance with the laws of porosity and pore size distribution (cf. Figure 6 and Table 4).
3.3 XRD and DSC-TG analysis
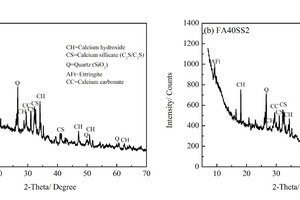
To facilitate discussion of the effects of SS on hydration products and microstructures of CHVFA pastes under steam curing, XRD patterns of samples with 0 % and 2 % SS are outlined in Figure 7. It can be seen that the types of hydration products of these four samples are fundamentally the same, including ettringite (AFt), calcium hydroxide (CH), calcium carbonate (CaCO3), quartz (SiO2) and unhydrated calcium silicate (CS).
It is generally known that CH reacts with silica in a supplementary cementing system and forms C-S-H, hence resulting in improved mechanical properties of mortars. On the XRD scale, the strongest peak of CH is located at about a 2-theta angle of 18.05, and in the past, the CH peak was considered to be the main indicator of performance in cement samples. For samples with SS, the CH peaks are weaker than those of the control sample, the reason being that the activity of FA is improved by the excitation effects of alkali and sulfate [9-11], and accordingly, the consumption of CH by FA would decrease the content of CH in the mixture. As for the CS peaks, the addition of SS promotes the hydration of cement, thus reducing the CS peaks significantly. Moreover, the addition of SS would cause a significant increase in AFt, because SS incorporation increases the amount of SO42-.
In general, both qualitative and quantitative analysis of C-S-H cannot be performed on the basis of XRD results, because C-S-H gel has an amorphous structure that does not reflect well in the XRD spectrum. Although there is no direct way to confirm the C-S-H concentration, the observed amount of CH can indirectly confirm the C-S-H concentration. Studies [34] pointed out that the content of CH shows a negative correlation with that of C-S-H, and if the amount of C-S-H increases, the intensity of CH peaks determined by XRD would decline [35]. In addition, the subsidence of CS peaks can also be a sign of the generation of C-S-H. Thus, lower CH and CS intensities in XRD of samples with SS show that more C-S-H has been generated. As a matter of fact, the increase in compressive strength is another piece of circumstantial evidence indicating C-S-H formation.
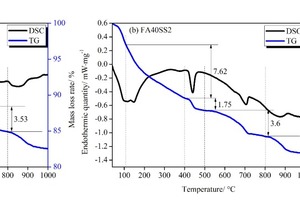
In order to quantitatively analyze CH, DSC-TG was used, and the results are shown in Figure 8 (a) and (b). In this study, there are five obvious endothermic peaks on all DSC curves from 40 to 1000° C, and correspondingly, five weight changes representing mass loss appear on the TG curves. Among them, the curve change between room temperature and 105° C is caused by the loss of free water. Between 105 and 400° C, this is caused by the loss of water from AFt and C-S-H gels. Between 400 and 500° C, it is caused by the dehydration of CH, between 500 and 800° C by the thermal decomposition of CaCO3, and, at temperatures higher than 800° C, by the ignition loss of cement and FA. According to Equation (1), the content of CH can be calculated as shown in Figure 8 (c).
As seen in the bar graph, SS incorporation decreases the CH content from 14.17 % to 13.26 %, mainly because FA consumes some CH under alkali and sulfate activation. All of these correspond with the results of XRD. In general, the lower CH content represents the higher C-S-H content, and correspondingly, the water loss in the sample with SS from 105° C to 400° C is higher than that without SS.
3.4 NMR and SEM analysis
The solid-state 29Si MAS NMR spectra of raw material are shown in Figure 9. The 29Si MAS NMR spectra corresponding to hydrated pastes are shown in Figure 10.
The assignments of 29Si NMR components are based on the previous study [28, 29, 31]. The peaks at about -71, -78, -81, -84, -99 and -107 correspond to Q0, Q1, Q2(Al), Q2, Q3 and Q4, respectively. As Figure 9 shows, there are two obvious peaks in each raw material sample, the sharp one being centered at -71 ppm, and the gentle one, which presents a wide asymmetric signal, is centered at -107 ppm. As shown in the literature [31], due to the overlap of peaks, Q3 and Q4, which represent the existence state of silicon oxygen tetrahedron in FA, are difficult to distinguish, while silica tetrahedron in cement existing in the form of Q0 is quite discernible.
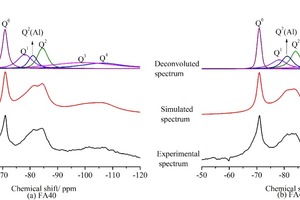
Because it is difficult to distinguish every peak in 29Si MAS NMR spectra due to the increasing forms of silica tetrahedron after cement and FA hydration, computer deconvolutions and peak-fit processing of the spectra were made as shown in Figure 10. Table 5 shows the integral intensity of Qn (n=0~4) peaks in each 29Si NMR spectrum. The hydration degree of cement and FA, and ACL and Al/Si ratio of C-S-H were calculated with Eqs. (2), (3), (4), (5).
Judging by the above results, the addition of SS could simultaneously accelerate the hydration of cement and FA and increase the ACL and the Al/Si ratio of C-S-H, compared to the control sample.
The increased degree of cement and FA hydration may favor improvement of the cement-based materials. For instance, SS incorporation can promote the hydration of cement and FA, a fact which is consistent with the results of XRD and DSC-TG, and as shown in Figure 4 and Figure 5, the compressive strength increases. The strength improvement also can be explained by the increase in the degree of silicate polymerization of C-S-H. In general, a higher value of ACL indicates a higher degree of polymerization of C-S-H, which is helpful for increasing the compressive strength of the cement matrix [34]. As seen in Table 5, the addition of SS increases the ACL of C-S-H significantly compared to that of the sample without SS, thus increasing the compressive strength of the mortars. The higher Al/Si ratio indicates that the addition of SS can promote the dissolution of Al.
To directly monitor the acceleration effect of SS on FA hydration, SEM was used to observe the hydration products on the FA surface, as shown in Figure 11.
As Figure 11 shows, FA in the control sample is no longer clean and smooth, and there are some hydration products coated onto the surface of spherical particles, showing that the activity of FA is stimulated under conditions of steam curing and high temperature, and that the secondary hydration reaction has already taken place. After mixing with SS, the corrosion in FA is more serious than in the control sample. Specifically, more hydration products are generated on the FA surface. This is mainly due to the double excitation of alkali and sulfate on FA [9-11], which accelerates the hydration of FA. All of this is consistent with the results of XRD, DSC-TG and NMR.
4 Conclusion
The main conclusions from this work are as follows:
(1) The addition of SS can accelerate both cement and fly ash hydration, decrease the porosity and pore volume, and increase the polymerization degree of C-S-H, thus enhancing the compressive strength of cement-based materials containing HVFA.
(2) The CH content decreases along with the SS incorporation, while the Al/Si ratio increases, indicating that the addition of SS can promote the dissolution of Al.
(3) The amount of C-S-H increases along with the addition of SS, specifically in the decrease of CH and CS, and the water loss in samples with SS, from 105° C to 400° C, is higher than that without SS.
5 Acknowledgment
The authors would like to acknowledge the 13th Five–Year Plan of National Key Research and Development (2016YFC0701003-05) and the Technology Support Program of Hubei Province (2015BAA084) for supporting this research and providing the test materials.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.