Simulation of limestone calcination in normal shaft kilns –
Part 3: Influence of particle size distribution and type of limestone
The decomposition process of individual particles with different particle size distributions and different types of limestone will be presented. Residual CO2 can only be found in the largest particles of the distribution. Small particles reach substantially higher core temperatures than large particles. Because of very high heat exchange through radiation, the surface temperatures of all particles are similar. Particles with lower thermal conductivity of the lime shell have lower temperatures and a higher amount of residual CO2 at the same process conditions.
1 Introduction
In the first part of this series, a mathematical model describing the simulation of burning limestone was presented [1]. In the second part examples were presented showing how the process parameters specific energy consumption, mean particle size and bed height effect the limestone decomposition [2]. The effect of the material properties particle size distribution and the type of limestone will be shown in this part. Two typical size distributions, 40 to 80 mm and 60 to120 mm, will be considered. Out of all the material properties, the thermal conductivity of the quicklime has...
1 Introduction
In the first part of this series, a mathematical model describing the simulation of burning limestone was presented [1]. In the second part examples were presented showing how the process parameters specific energy consumption, mean particle size and bed height effect the limestone decomposition [2]. The effect of the material properties particle size distribution and the type of limestone will be shown in this part. Two typical size distributions, 40 to 80 mm and 60 to120 mm, will be considered. Out of all the material properties, the thermal conductivity of the quicklime has the largest effect on the decomposition time. Depending on the origin of the limestone, this can vary from 0.4 to 0.8 W/m/K. That is why the change in temperature and the decomposition of two limestone samples with different conductivities will be compared. The presented simulation program can be used for further variation of parameters.
2 Particle size distribution
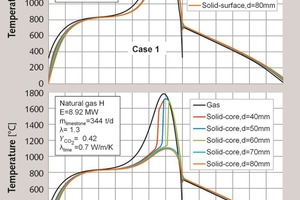
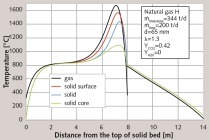
First, a particle size from 40 to 80 mm will be considered. The distribution of particle size can usually be effectively described with the Rosin-Rammler Correlation. However, in this case the distribution was separated into five classes, 40, 50, 60, 70, and 80 mm. Each class was composed of the same percentage of the total mass in order to simplify the explanation of influences. In Figure 1 the axial profiles of surface temperature of the stone (above) and core temperature of the stone (below) are shown for each class. Additionally, each figure also contains the gas temperature whereas the process conditions are shown in the legend.
It is apparent that the smaller particles heat up faster than the larger particles. This occurs because the convective heat transfer coefficient decreases with diameter at a power of 0.5. During the decomposition process, the surface temperatures of the stones are similar. This occurs because there is a high rate of heat transfer between the stones through radiation. Because of the very high temperatures, there is a high amount of radiation despite the relatively low emissivity of lime (0.3 to 0.6). The small stones receive more heat through convection than the large stones. The small stones then immediately radiate the extra heat to the larger stones. For this reason, larger stones in polydisperse systems decay faster than in monodisperse systems. The gas temperature is not substantially higher than the surface temperature because the heat transfer in solid beds is relatively high.
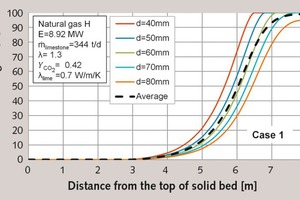
The core temperature is considerably different between the different classes. Figure 2 shows the relevant decomposition profiles. The smallest class (40 mm) was the first to decompose after approximately 6.5 m. At that point, the core temperature increased until it reached the surface temperature. After 6.8 m, the second class (50 mm) decomposed and the core temperature also increased with a steep slope. The 60 mm class behaved similarly. The 70 mm class decomposed after 7.8 m. For this reason, the core temperature for the 70 mm class did not reach as high values as the smaller classes. The 80 mm stones did not fully decompose and the core of these stones had a considerably lower temperature than the other stones.
In Table 1, the characteristic values for the five classes are summarized. The throughput was, as predicted, 68.8 t/d for all classes. Only the 80 mm stones did not fully decompose and had a residual CO2-content of 3.62 %. Therefore the lime output was slightly larger than the smaller classes. The lime output temperature was even lower, when the stones were smaller. This is, as mentioned before, due to the higher convective heat transfer. The maximum surface temperature did not differentiate substantially between the different particle sizes. The core temperatures, on the other hand, were considerably different. This could result in differences in the reactivity of the different classes.
In the first column of Table 2, the characteristic values of the process for the first case are given, including specific energy consumption, pressure loss, etc. These values have already been discussed in the previous parts of the article series. The residual CO2-content of the complete process amounts to 1/5 of the residual CO2-content for the 80 mm particle.
In the second case, the particle size distribution was reduced, so that the 40 mm stones are eliminated. The mass percent of the other four particle sizes are still equal at 25 %. Figure 3 shows the axial profile of the core temperature of the stones and the gas temperature. The 50 mm particles decomposed at 6.8 m again, as before, whereas the core temperature also increased steeply. However, it is noticeable that the maximum temperature is approximately 70 K less than in the previous case. So it could be observed that small particles increase the maximum temperature through their faster decomposition.
In Table 3, the characteristic data for the four classes are summarized. It is apparent, that the 70 mm particles also did not completely decompose. The 80 mm particles contained 4.05 % higher residual CO2-content than in the previous case. The average values for this process are shown in Table 2. The total residual CO2-content increased. In the previous part of this series, it was found that larger stones with the same process conditions result in a higher residual CO2-content. This could be reduced through higher energy input or a reduction of limestone throughput.
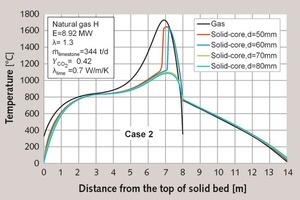
In the third case, a distribution of particle size from 60 to 120 mm was used. Figure 4 shows the axial profiles of core temperature and gas temperature. The process conditions in the legend are the same as those in the first two cases. It is of note, that the maximum temperature is approximately 50 K lower than the 40 to 80 mm distribution, and also that the difference between stone and gas temperature is higher.
The characteristic values for the five classes are given in Table 4, and the average process data are given in Table 2. The 120 mm particles contain 14.21 % residual CO2, compared to 5.19 % for the whole solid bed. The CO2-content can be reduced with a higher energy input.
In the following, the effect of particle size distribution will be discussed. In Tables 5 and 2, the characteristic data from the fourth case is presented, in which the distribution (75 to 120 mm) is tighter. By analogy to the second case, the smallest class (here 60 mm) was removed. The residual CO2-content increased to 6.99 % and the maximum temperature was lower by around 100 K.
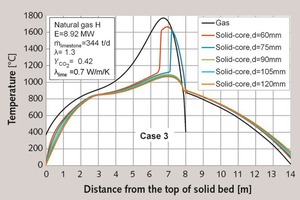
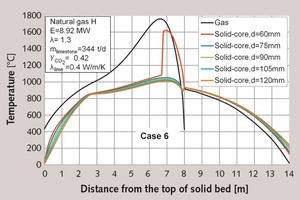
In Tables 6 and 2, the fifth case is shown, in which the distribution was from 60 to 105 mm. In this case, the 120 mm particles were removed. Because of this, the residual CO2-content was significantly reduced. The maximum temperatures increased as before, so that a faster decomposition was possible.
From these changes in the distribution it can be concluded, that under the same process conditions, the residual CO2-content can only be reduced through a reduction of the larger stones or through a higher energy input.
3 Effect of limestone type
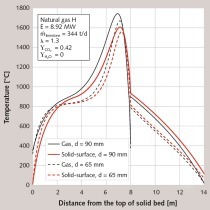
In the last case, the effect of the origin of the limestone was investigated. The crucial parameter in this case is the thermal conductivity of the quick lime. In Figure 5 the axial profiles of the core temperature and the gas temperature for the 60/120 distribution are presented. Here, the quick lime has a relatively low thermal conductivity of 0.4 W/m/K. From Table 7 and Table 2 it is clear that only the smallest class of 60 mm fully decomposed. The total residual CO2-content increased to a high value of 9.93 %. The temperature level degraded substantially, so that a high temperature difference is required for heat transfer. In order to reduce the residual CO2-content, the energy input must be increased or the stone throughput must be decreased.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.