Mechanical properties and hydration characteristics of sulfoaluminate cement-based materials containing nano silica
This paper examines the effect of nano silica (NS) on the mechanical properties and hydration characteristics of materials based on sulfoaluminate cement (SAC). The compressive strength and flexural strength of mortars were measured with different NS addition levels ranging from 0 to 3%. The results indicate that the compressive strength and flexural strength are significantly improved with increasing NS content up to 2% and then decrease slightly with a further increase in NS content (e.g. at 3%). The strength loss of SAC-based materials can also be avoided when NS is added. The underlying mechanism was then analyzed by mercury intrusion porosimetry (MIP), microcalorimetry, X-ray diffraction (XRD), differential thermogravimetry (DTG), nuclear magnetic resonance (NMR) and scanning electron microscopy (SEM). The results indicate that the microaggregate filling effect and nucleation effect of NS act as the major factors in the early stages while the pozzolanic effect plays a dominant role in the later stages. These effects are beneficial in improving the compactness of SAC-based materials, and thus in increasing the strength and avoiding the strength loss at all ages. These results could also be expected to provide guidance on the use of NS in SAC-based materials.
1 Introduction
Sulfoaluminate cement (SAC) has been regarded as a green and environment-friendly high-performance special cement due to its lower energy consumption and lower CO2 emissions when compared with OPC production [1, 2]. This provides very optimistic prospects for development. Due to the advantages of rapid setting time, high early strength, negative temperature hardening and low alkalinity [3, 4], SAC has also been widely used in repair engineering and many special projects. However, it cannot be ignored that sulfoaluminate cement has the defect of reduced flexural strength [5]. When...
1 Introduction
Sulfoaluminate cement (SAC) has been regarded as a green and environment-friendly high-performance special cement due to its lower energy consumption and lower CO2 emissions when compared with OPC production [1, 2]. This provides very optimistic prospects for development. Due to the advantages of rapid setting time, high early strength, negative temperature hardening and low alkalinity [3, 4], SAC has also been widely used in repair engineering and many special projects. However, it cannot be ignored that sulfoaluminate cement has the defect of reduced flexural strength [5]. When sulfoaluminate cement is used in structural engineering the existence of this defect may lead to the destruction of beams, plates and other structural parts or even, under certain circumstances, of the overall structure, thereby endangering the safety of life and property.
With the rapid development of nanotechnology in the past 10 to 20 years, many domestic and foreign scholars have studied the effects of nano materials, especially nano silica (NS), on the hydration of Portland cement [6-10]. In general, NS can provide a crystallization nucleation point for hydration products such as ettringite, thereby controlling the decomposition and transformation ettringite. In addition, it can promote the formation of C-S-H gel by reacting with CH [7], so that the hardened cement paste becomes denser [8, 9] with a corresponding improvement in the physical and mechanical properties and durability of cement-based materials [10]. Other studies have shown that the nano particles absorb more free water due to the large specific surface area during agitation, which reduces the workability of the fresh paste [11]. At present, these studies have mainly focused on the impact on Portland cement and, due to the different mineral composition, there is still much controversy about whether or not such nano effects also occur with SAC. These problems concerning whether nano materials improve the mechanical properties and hydration characteristics of SAC-based materials therefore need to be studied further.
This study investigated the influence of NS on the mechanical properties and hydration characteristics of SAC-based materials. The compressive strengths and flexural strengths were measured in order to evaluate the mechanical performance. The pore structure was studied by MIP, and the effect of NS on hydration was examined using heat of hydration, XRD, DTG, 29Al-NMR and SEM. These investigations revealed the mechanism behind the improvement in mechanical properties in terms of hydration and pore structure. These results could be expected to provide guidance in promoting the use of NS in SAC-based materials.
2. Raw materials and experimental processes
2.1 Raw materials
The low alkalinity, 42.5 grade, SAC produced by Deng’feng Wang’lou Cement Plant has a specific surface area of 427 m2/kg and a specific gravity of 2.87. The nano silica (NS) produced by Shang’hai Aladdin Biochemical Technology Co., Ltd. is a white amorphous powder with a specific surface area of 200 m2/g and an average particle size of 15 nm. A polycarboxylate superplasticizer (SP) produced in the laboratory was used to ensure the same fluidity level with the same water/binder ratio. Standard sand produced in Xia’men of China in accordance with GB/T14684-2011 [12] was used as the aggregate for the mortars. Deionized water was used throughout the experiment.
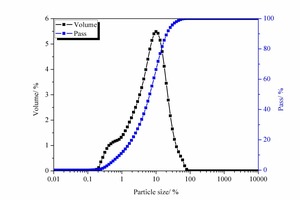
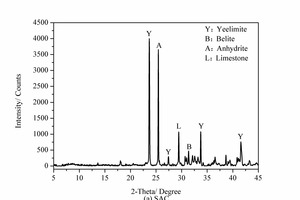
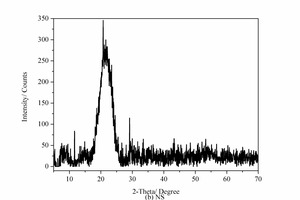
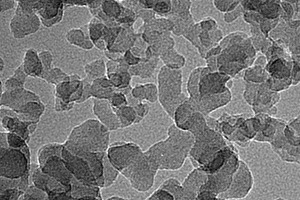
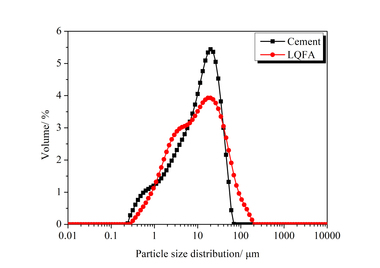
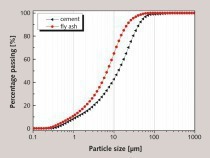
The chemical composition of the SAC, obtained with an X-ray fluorescence spectrometer (XRF, Axios advanced), is shown in Table 1. The particle size distribution of the SAC was tested with a Mastersizer 2000 laser particle size distribution instrument and the results are shown in Figure 1. The phase constitution and microstructure were examined by XRD and TEM respectively, as shown in Figure 2 and Figure 3. The XRD patterns indicate that the NS is an amorphous material and is relatively less crystalline than the SAC. The TEM image confirms that the particle size of the NS is about 15 nm.
2.2 Mix proportions and sample preparation
The mix ratios of the mortars and pastes are shown in Table 2.
The mortars were cast in accordance with GB/T17671-1999 [13] in prismatic moulds (40×40×160 mm3) with a sand-binder ratio of 3.0 and a water-binder ratio of 0.5 by weight to study the influence of the NS on mechanical performance. The addition levels of NS, sand, and SP were related to the total mass of SAC and NS, as shown in Table 2. After curing in a chamber with a relative humidity above 90% and a temperature of 20±1 °C for 2 h, 8 h,1 d, 3 d, 28 d or 90 d, the mortar samples were prepared for measurement of the 2 h, 8 h,1 d, 3 d 28 d and 90 d strengths.
The pastes were prepared with the same mix proportions as the mortars, but without sand, and cast in moulds (40×40×40 mm3) to study the hydration process and microstructure. The curing process for the paste samples was the same as above and small pieces of these pastes were then immersed in anhydrous ethanol in order to stop their hydration. Some of the small pieces were used for measurement of the pore structure and for SEM and some were ground to powder that could pass a 200 mesh sieve for the XRD, DTG, and NMR measurements.
It can also be seen from Table 2 that if the fluidity is basically consistent the content of SP increases with increasing NS content, indicating that the addition of NS has an adverse effect on the fluidity of SAC-based materials. This is mainly because the nano particles with their high special surface area can absorb a great deal of free water [11].
2.3 Test procedure
2.3.1 Strength
The flexural strength and compressive strength were measured in accordance with GB/T17671-1999 [13] using a WYA-300 series fully automatic test machine. Three samples for flexural strength and six samples for compressive strength were tested for each mix and the average taken as the result.
2.3.2 Pore structure
The pore structure of the sample was evaluated using a Quantachrome Autoscan-60 mercury intrusion porosimeter with a maximum pressure of 300 MPa and a contact angle of 140 °. The results were analyzed using Pore Master, Excel and Origin software.
2.3.3 XRD analysis
Phase analysis was performed using a Bruker D8 Advance X-ray diffractometer with a Cu kα spectrum. The working voltage and working current used during the test were 35 kV and 30 mA respectively. When the data was being collected the step-length was 0.02 °/s, the scanning rate was 8 °/min, and the 2-theta angle ranged from 5 to 45 °.
2.3.4 DTG analysis
A Netzsch STA449F3 simultaneous thermal analyzer was used for the DTG analysis, which was carried out from 40 °C to 1000 °C at a heating rate of 10 °C/min in an atmosphere of nitrogen.
2.3.5 27Al MAS-NMR analysis
Solid-state 27Si magic-angle spinning nuclear magnetic resonance (MAS-NMR, Bruker Avance III 400) was used to analyze the composition and structure of Al-based substances. A 5 mm zirconia rotor was used during the test with a rotation frequency of 12000 HZ and a relaxation time of 5 s.
2.3.6 SEM analysis
Quanta 200 FEG-SEM with a 5 kV accelerating voltage and 10 mm working distance in low vacuum mode was used for intuitive observation of the morphology characteristics of the SAC-based materials.
3 Results and discussion
3.1 Strength
Table 3 shows the development of the compressive and flexural strengths of SAC mortars with different addition levels of NS.
Table 3 shows that the compressive and flexural strengths of SAC mortars can be improved to differing extents by the addition of NS. When there is no NS the early compressive strength develops rapidly and the compressive strength at 3 d reaches 90.6% of that at 28 d and 88.0% of that at 90 d. However, the compressive strength of pure SAC mortar develops slowly from 28 d to 90 d and only increases by 2.9%. When NS is included the compressive strength at all ages is higher than that of the control sample and, in particular, there is still a significant increase in the late strength. Specifically, the compressive strengths of the NS1, NS2 and NS3 samples increase from 28 d to 90 d by 7.9%, 10.2% and 8.3% respectively.
Table 3 also shows that the flexural strength of pure SAC mortar is reduced at later ages. For example, the flexural strength of the control sample at 90 d is 3.6% lower than at 28 d. On the other hand, 1%, 2% and 3% NS cause the flexural strength to increase by 6.9%, 9.2% and 7.4% respectively, indicating that inclusion of NS solves the problem of late flexural strength reduction of SAC-based materials. This may be connected with the alkalinity of the system. In fact, some research has found that the swelling and destruction associated with ettringite, thus causing micro-cracks in the hardened cement paste structure, is more likely to occur in SAC systems with higher alkalinity [14, 15]. The calcium hydroxide (CH) can be adsorbed continuously when NS is incorporated, thereby reducing the alkalinity of the system. The ettringite produced under low alkalinity undergoes less expansion, which reduces the microcracks caused by the expansion with the result that the collapse in flexural strength does not appear in SAC mortar containing NS.
It is also clear that much higher compressive and flexural strengths than those of the control sample can be obtained at all ages with the addition of 3% NS in spite of the slightly lower strength than those with 2% NS. The addition of NS can therefore increase the strength, with the optimum at 2%. The slight decline in strength of mortar with the addition of 3% NS is attributed to agglomeration of the nano particles accompanied by a decrease in specific surface area of the NS [16, 17], resulting in a slight decline in the nano effect of the NS.
3.2 Pore structure
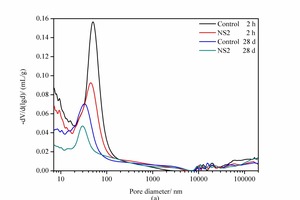
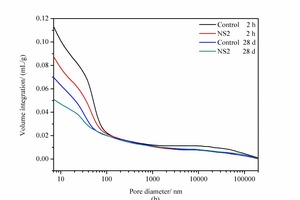
Cement paste is a multi-component structure system that includes crystals, gels, liquids, pores, etc. The pore structure, which has an important influence on the macroscopic properties of cement-based materials, involves the porosity, pore-size distribution and pore geometry. According to research by Wu [18], the pores in cementitious composites can be roughly classified into four categories: harmless pores (smaller than 20 nm), slightly-harmful pores (between 20 and 50 nm), harmful pores (between 50 and 200 nm) and more-harmful pores (larger than 200 nm). The results for the pore structure of the control sample and the NS2 sample at 2 h and 28 d are shown in Figure 4 and Table 4.
As shown in Figure 4 (a), it is worth noting that there is one major peak for each derivative curve of pore size distribution. At 2 h the peak value for the addition of 2% NS has decreased from 50.43 nm to 45.17 nm (see Table 4), which indicates that NS with small nanometre sizes can cause a significant reduction in the most probable aperture. As shown in Table 4 (2 h samples), the pores larger than 200 nm remain basically unchanged, while the pore sizes smaller than 20 nm, between 20 and 50 nm, and between 50 and 200 nm show a remarkable decrease. This demonstrates that NS can reduce the porosity, the total pore volume and the harmful pores, with a clear effect on refining the pore structure. This might be related to the crystal nucleus effect and micro-aggregate effect of NS.
When the curing time is increased to 28 d it is clear that the porosity and total pore volume of all the samples are lower than those at 2 h. This is because the hydration products are formed more gradually with increasing curing time, which allows the pores to be filled and the paste to become much denser. Furthermore, the porosity, the total pore volume and the pore size distribution for samples at 28 d have the same trend in changes as those at 2 h, and the porosity as well as the most probable pore size of the NS2 sample are also lower than those of the control sample.
The results show that the addition of NS can significantly reduce the porosity, optimize the pore size distribution and increase the compactness of the hardened cement paste, thus improving the strength of cement-based materials, which is in agreement with the results listed in Table 3.
3.3 Heat of hydration
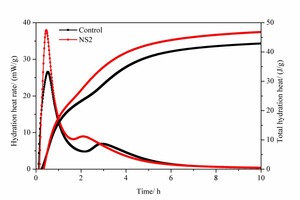
Figure 5 shows the hydration heat release rate and total heat release curves over time for pure SAC paste and for the 2% NS-modified SAC paste.
Two distinct exothermic peaks appear in the SAC pastes in Figure 5, regardless of whether or not NS is included. These are due to the ettringite formed at different stages. The difference is that the addition of NS advances the time of the exothermic peak and accelerates the rate of release of the heat of hydration. Specifically, after the inclusion of NS the first and second exothermic peaks were 5 min and 50 min earlier than those of the control sample and the first peak increases from 26.55 mW·g-1 to 37.94 mW·g-1, and the second peak increases from 6.93 mW·g-1 to 8.916 mW·g-1. The reason for this phenomenon is that the nano materials can provide crystallization nucleation points for the growth of the hydration product of ettringite, thereby promoting the crystallization of ettringite and correspondingly accelerating the overall hydration of cement.
From the curves of total hydration heat it can be seen that up to 1 h, the total hydration heat curve of the sample with NS is basically consistent with that of the control sample. However, from 1 to 10 h, the total hydration heat of the sample with NS exceeds that of the control sample, especially between 2 h and 10 h. This indicates that the inclusion of NS can promote the early hydration of cement.
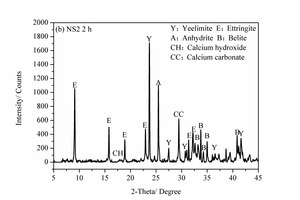
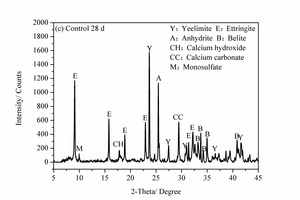
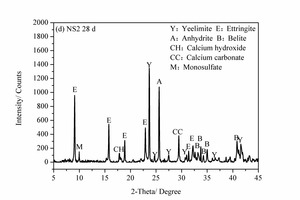
3.4 XRD analysis
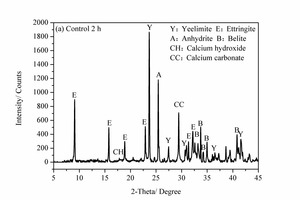
Figure 6 shows the XRD images of the pure SAC paste and the 2% NS-modified SAC paste at 2 h and 28 d. It can be seen that, for the samples at 2 h, the main hydration products are ettringite (AFt), anhydrite (CaSO4), calcium hydroxide (CH) and calcium carbonate (CaCO3) as well as unhydrated yeelimite (C4A3S̅) and belite (β-C2S). However, for the samples at 28 d some monosulfate (AFm) is also formed in addition to the above products.
As shown in Figure 6 (a) and (b), the AFt diffraction peak of the NS2 sample at 2 h is higher than that of control sample, indicating that the degree of crystallization of the AFt in the NS2 sample is greater than that in the control sample. A large amount of C4A3S̅ is also consumed at the same time, which may be because the NS can provide crystalline nucleation sites for hydration products at the early stages, thus consuming more clinker [7, 19].
Unhydrated C4A3S̅ and β-C2S clinker minerals were still present in all he samples after hydration for 28 d but the diffraction peak intensities were somewhat lowered. This shows that the cement hydration continues with time and so more clinker is consumed. For the sample mixed with NS the XRD diffraction peak for AFt was lower than that of the control sample, while the diffraction peak for AFm was greater than that of control sample. This is probably because the heterogeneous nucleation effect of NS promotes early hydration of SAC, making the cement paste more compact and causing more sulfate to be in solid solution in the hydration products. This reduces the SO42- concentration in the system and leads to the transition from AFt to AFm [20]. C2S also starts to react at the later ages and a small amount of CH is formed. CH can be consumed by NS due to its high pozzolanic activity, thereby promoting the hydration of β-C2S. Furthermore, the reduction of CH in the NS2 sample lowers the alkalinity of the system, which is beneficial to the volume stability of AFt and thus prevents the strength from decreasing over time. This can be summarized by stating that the inclusion of NS causes greater consumption of C4A3S̅ and β-C2S after hydration for 28 d due to the heterogeneous nucleation effect and pozzolanic effect of the NS, and there is a corresponding lowering of the diffraction peak intensities.
Based on these discussions it can be concluded that NS accelerates the hydration of SAC at both 2 h and 28 d, and also decreases the alkalinity of the system, thus increasing the compressive and flexural strengths.
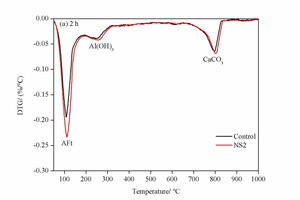
3.5 DTG analysis
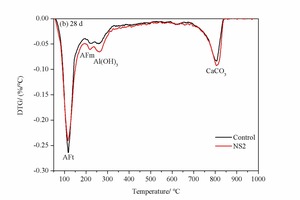
Figure 7 shows the DTG analysis of the control sample and NS2 sample at 2 h and 28 d. The decomposition peaks of AFt, AFm, Al(OH)3 and CaCO3 are marked in the DTG patterns [21]. There are obvious characteristic peaks of Al(OH)3 at the age of 2 h in Figure 7 (a), which differs from the XRD data with no clear Al(OH)3 peaks. This is mainly because Al(OH)3 is an amorphous substance with poor crystallinity, which is does not show up well in the XRD pattern. The AFt peak in NS2 is also higher than that in control sample, indicating that more clinker is hydrated and that there is an increased content of AFt. This is in accordance with the XRD results.
When the curing age is extended to 28 d, as shown in Figure 7 (b), AFm peaks can also be observed in the control and NS2 samples in addition to the AFt, Al(OH)3 and CaCO3 peaks. In contrast to the samples at 2 h the AFt peak in the NS2 sample is lower than that in control sample, while the AFm peak in the NS2 sample is higher. This indicates that the addition of NS favours the conversion of AFt to AFm, which is in agreement with the result obtained by XRD.
3.6 NMR analysis
It is well known that the use of 29Si and 27Al NMR technology to characterize the structure of hydration products is currently a hot research topic in cement chemistry. 27Al is used in this section to describe the influence of NS on the AFt transformation in SAC-based materials. According to the literature [21], the Al-O of C4A3S̅ in SAC has a coordination number of 4. It reacts with gypsum and β-C2S to form AFt and AFm where the Al-O has a coordination number of 6, i.e. the SAC hydration process is a transformation process of the Al-O structure from 4-coordination to 6-coordination. The chemical shift value of the Al-O gradually decreases with increasing hydration.
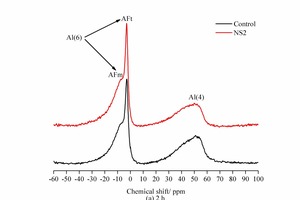
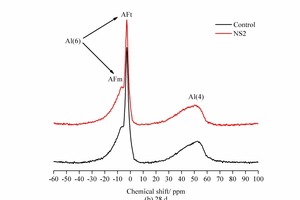
Figure 8 shows the 27Al NMR spectra of the hydration products of SAC at 2 h and 28 d.
As shown in Figure 8, there are two Al-O characteristic peaks with 6-coordination (Al (6)), i.e. the AFt phase with a chemical shift around -3 ppm and the AFm phase with a chemical shift around -6 ppm. The characteristic peak of the AFt is clear at both 2 h and 28 d while the AFm peak is less conspicuous. The AFt characteristic peak at 2 h is more significant in the NS2 sample than in the control sample, while the peak marked as Al(4) in the NS2 sample is lower, indicating that the addition of NS favours the formation of AFt. This is mainly because the NS can be used for heterogeneous nucleation to accelerate the consumption of C4A3S̅, which is advantageous to the crystallization and precipitation of AFt. Although the Al(4) peak in the NS2 sample at 28 d is also lower in the control sample, the lower characteristic peak value of AFt and higher characteristic peak of AFm indicates that the addition of NS is conducive to the transformation of AFt to AFm. The results from the NMR are in line with those from XRD and DTG.
3.7 SEM analysis
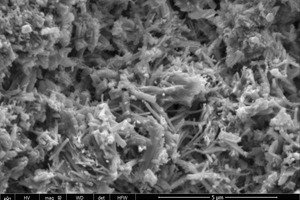
Figure 9 shows the SEM images of the hydration products from the control sample and NS2 sample at 2 h and 28 d.
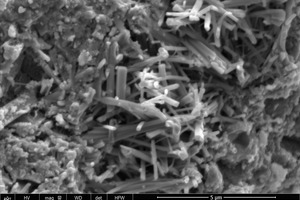
As shown in Figure 9 (a) the control sample at 2 h is mainly composed of unhydrated clinker particles, AFt, and a large number of pores. The microstructure of the AFt is clearly visible as needle crystals. When 2% NS is added (see Figure 9 (b)) it is clear that there is a reduction in the unhydrated clinker particles, the AFt crystals become noticeably thicker and the microstructure is more compact, resulting in an increase in the compressive and flexure strengths. All of this can be attributed to the heterogeneous nucleation effect and the microaggregate filling effect of the NS.
A great deal of C-S-H gel appears when the curing time is increased to 28 d and the AFt consists mainly of thicker rod-like crystals, regardless of whether the control sample or the NS2 sample is involved. There is also a clear improvement in the density of the microstructure. All this makes the samples stronger as the hydration progresses.
Comparison of the samples with and without nano particles at 28 d (see Figure 9 (c) and (d)) shows that the addition of NS results in more C-S-H gel products and thicker AFt crystals. This is mainly because the pozzolanic effect of the NS promotes the consumption of CH, which is beneficial to the hydration of β-C2S. There is a greater tendency of the gels to crisscross and interweave from different directions with the AFt, thus forming a denser structure and leading to higher compressive and flexural strengths. This is consistent with the results obtained from Table 3.
4 Conclusion
The main conclusions that can be drawn from this study are as follows:
The addition of NS can accelerate the hydration of SAC, decrease the porosity and total pore volume, optimize the pore size distribution and lower the alkalinity of the system. This enhances the compressive and flexural strengths and at the same time avoids a loss in strength of SAC-based materials.
The microaggregate filling effect and nucleation effect of NS are the major factors for improving the strength of SAC-based materials at 2 h, while the pozzolanic effect plays a dominant role at 28 d.
The inclusion of NS is beneficial to the formation of AFt at 2 h, while at 28 d there is a slight decline in the amount of AFt but an obvious increase in AFm, indicating that NS is beneficial to the transformation of AFt to AFm.
As the hydration progresses the microstructure of the AFt changes from fine needles to thick rods, and more C-S-H gel products and thicker AFt crystals are formed when NS is added.
Acknowledgement
The authors would like to acknowledge the assistance of the National Natural Science Foundation of China (51908434), the Open Foundation of State Key Laboratory of Silicate Materials for Architectures of Wuhan University of Technology (SYSJJ2018-14) and the National Key R&D Program of China (2016YFC0701003-5).

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.