Influence of nano-TiO2 on the rheological and fresh properties of sulphoaluminate cement-based materials
The influence of nano-TiO2 (NT) on the rheological parameters, fluidity, setting time and pore structure of sulphoaluminate cement (SAC) composites is studied and the influence mechanisms are investigated by hydration heat, X-ray diffraction (XRD) and differential scanning calorimetry-thermogravimetry (DSC-TG). Results show that the addition of NT increases the apparent viscosity, shear stress and apparent density of SAC paste while decreasing its fluidity, total pore volume, average pore size and porosity and shortening its setting time. In addition, incorporating NT promotes the early hydration of SAC and accelerates the formation and precipitation of hydration products. Then, the hydration geometric model is established and visually reveals the internal structure development process of NT-modified SAC paste.
1 Introduction
For the past few years, the application of nano-particles in the field of construction and building materials has been receiving more and more attention. When cementitious composites are modified by superfine particles, functional products or materials with different characteristics from traditional materials are gained [1-8]. Many studies have shown that the mechanical properties, working performance and durability of cement-based materials can be improved to a greater or lesser extent by adding nano-particles.
As a new type of function material, nano-TiO2 has a wide range of...
1 Introduction
For the past few years, the application of nano-particles in the field of construction and building materials has been receiving more and more attention. When cementitious composites are modified by superfine particles, functional products or materials with different characteristics from traditional materials are gained [1-8]. Many studies have shown that the mechanical properties, working performance and durability of cement-based materials can be improved to a greater or lesser extent by adding nano-particles.
As a new type of function material, nano-TiO2 has a wide range of applications for its multiple advantages, such as sewage disposal, air purification, antisepsis, anti-fog and self-cleaning, etc. As for construction materials, nano-TiO2 cannot only be used in making self-cleaning concrete, but also may provide additional nucleation sites for hydration products [9] and, in addition, affect the fundamental performance of cement-based materials [4]. Until now, however, though there has been much research on the influence of nano-TiO2 on Portland cement, little work has been done on sulphoaluminate cement, especially regarding its rheological and fresh properties. In addition, it is still controversial whether nano-TiO2 has a similar influence on sulphoaluminate cement compared to that of Portland cement.
In sulphoaluminate cement manufacturing, carbon dioxide emissions are reduced by 50 % compared to Portland cement production. Moreover, the manufacture of sulphoaluminate cement can consume both a lot of low-grade raw materials of other industries and by-products such as fly ash and slag, etc. Therefore, sulphoaluminate cement is a kind of environment-friendly, green, high-performance, special cement, and also enjoys promising prospects [10, 11]. With the advantages of fast setting time, high initial strength, negative temperature hardening and low alkalinity [12, 13], sulphoaluminate cement has been widely used in repair work and numerous specific engineering applications. However, the unstable strength development and high-temperature sensitivity of ettringite affect the basic stability of sulphoaluminate cement-based materials [14], a fact which strongly limits their wider application. So it is of theoretical and practical significance to study the influence of ultrafine powder and nanomaterials on sulphoaluminate cement properties.
The early strength development of hardened slurry is often affected by the rheological and fresh properties of sulphoaluminate cement. Thus, in this paper, the influence of NT on the rheological and fresh performance of SAC is studied. Moreover, the governing mechanisms of NT on these properties of sulphoaluminate cement are studied and demonstrated.
2 Experimental procedure
2.1 Materials
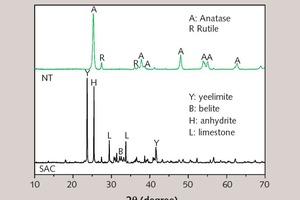
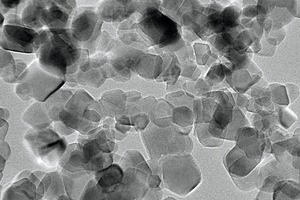
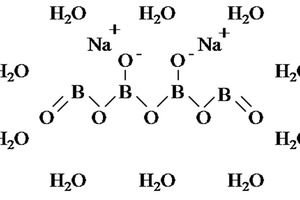
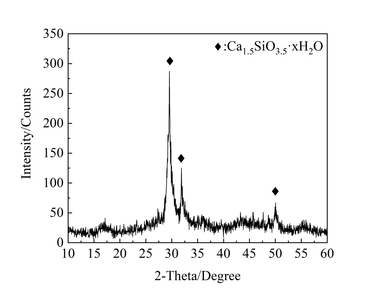
A sample of sulphoaluminate cement (SAC) with a specific gravity of 2.98 and a specific surface area of 0.41 m2/g was produced in the Dengfeng Wanglou cement plant, and its chemical composition and physical properties are shown in Table 1 and Table 2. The white crystalline powder of nano titanium dioxide (NT) with a specific surface area of 50 m²/g was obtained from Degussa Chemical Co., Ltd. XRD patterns of SAC and NT are shown in Figure 1. A transmission electron microscopy (TEM) image of NT is shown in Figure 2. 2016F-type polycarboxylate superplasticizer (PS) with a solid content of 40 % was purchased from BASF China Ltd. Sodium borate (SB) was used as the SAC setting retarder and its molecular structure is shown in Figure 3. The tap water accords with the level of mixing water in JGJ 63-2006 [15].
2.2 Mix proportion and sample preparation
Cement pastes were mixed with a water-binder ratio of 0.5, with NT substituting equal amounts of cement at 0 %, 1 %, 2 %, 3 %, 4 % or 5 % (mass fraction) and PS and SB accounting for 1 % and 0.8 % of binders, respectively. Their detailed mix proportions are listed in Table 3. Prior to mix preparation, NT was added to deionized water, stirred and dispersed by ultrasonication at 325 W for 30 min to obtain uniform suspensions.
All specimens for X-Ray Diffraction (XRD), differential scanning calorimetry-thermogravimetry (TG-DSC) and mercury intrusion porosimetry (MIP) were molded into squares with a size of 40 x 40 x 40 mm using SAC pastes. After curing to the test age, the hydration of the pastes was stopped by submerging the center part of the crushed samples in acetone solution. The samples were oven-dried at 80 °C for 4 h, and part of the block sample was used for MIP testing, while the others were hand ground in an agate mortar so as to pass the 100 mesh size sieve. The powder sample was used for XRD and DSC-TG testing.
2.3 Testing procedure
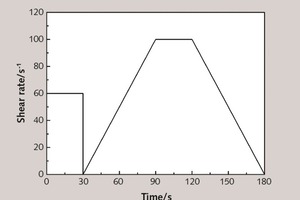
The rheological behavior of the paste was tested with a rheometer R/S-SST2000 produced in Brookfield. The rotor rotation program is shown in Figure 4. In the early stages, the fresh paste was stirred at a shear rate of 60 s-1 for 30 s, then the rotor stopped and the rate then increased linearly from 0 to 100 s-1 in 60 s, held there for 30 s, and finally linearly decreased to 0 in 60 s.
The fluidity experiment of SAC paste was conducted according to GB/50448-2015 [16] and the setting time tests were carried out according to GB/T 1346-2011 [17].
The heat evolution of pastes was conducted using a micro calorimeter C80 at a test temperature of 25 °C and a sample quality of 0.5 g. The composition and chemical phase analysis were confirmed using a Bruker D8 Advance XRD device with a Cu kα X-ray source at 40 kV and 40 mA. During data collection, the step-length was 0.02°, the scanning rate was 2 °/min, and the 2θ range was 5-70°. DSC-TG analysis was conducted using an STA449C/3/G apparatus at a heating rate of 15 °C/min from 50 °C to 900 °C in a nitrogen atmosphere. Pore structures of SAC paste were tested by Quantachrome AUTOSCAN-60 mercury intrusion porosimetry (MIP) with measurable aperture ranging from 3 nm to 360 μm, a maximum pressure of 300 MPa and a contact angle of 130 °.
3 Results and discussion
3.1 Rheological behavior
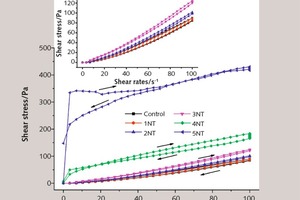
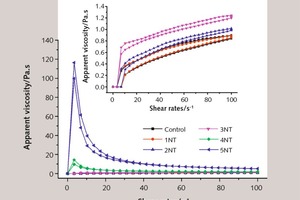
Figure 5 and Figure 6 show the influence of different dosage of NT on the shear stress and apparent viscosity of SAC pastes, respectively.
As can be seen from Figure 5, the shear stress increases along with the dosage of NT when the shear rate remains unchanged. Especially if the mixing content is above 3 %, the increasing magnitudes are more significant compared to the control sample. For example, when the shear rate is 50 s-1, the shear stress of sample 5NT is roughly 10 times larger than that of the control sample, mainly because the microstructure of the paste becomes more compact as the NT content increases, resulting in higher shear stress during stirring.
In addition, the shear stress of all samples increases along with the shear rate. Especially when the rate is below 3.45 s-1, the increment is more obvious, while when the rate is above 3.45 s-1, the stress increases more slowly. This is mainly because, along with hydration, the accumulating products of flocculent structure lead to greater and greater shear resistance. However, the degree of flocculent structure being damaged also increases along with the shear rate, thus decelerating the increase in shear stress.
The main characteristic of the rheological curve is the formation of a hysteresis loop, which can be used to evaluate the thixotropic property of the fresh paste. Specifically, the larger the area of the hysteresis loop, the higher the thixotropy, that is to say, the fluidity of the paste is rather changeable over time [18]. The paste with 5 % NT has the largest hysteresis loop area, indicating that the fluidity of sample 5NT changes noticeably with the passing of time. That may be because NT can provide nucleation sites for hydration products in the early stage of hydration [1, 4], and a large addition of NT will cause SAC paste to form more hydration products of flocculent structure. As the shear rate changes, numerous flocculent structures are destroyed, and the remaining weak strength flocculent structures and free particles make the rotor encounter little resistance in the process of stirring, so the shear stress is accordingly low.
As shown in Figure 6, at the same shear rate, the apparent viscosity of SAC paste increases in line with an increase in NT. In addition, when the shear rate changes, the variation extent of apparent viscosity of samples with 4 % or 5 % NT is more pronounced, and changes in other samples are more muted. Studies show that the specific surface area of solid particles is larger, and the apparent viscosity of fresh paste will be higher [19, 20]. Since NT has a larger specific surface area compared to that of SAC particles, it can absorb free water of mixture more effectively, which leads to a larger degree of overlap among solid phase in the paste, and accordingly higher apparent viscosity. In conclusion, for an increasing NT content, the thixotropy of fresh pastes becomes larger and larger, and the extent of apparent viscosity variation becomes more significant over time.
3.2 Fluidity
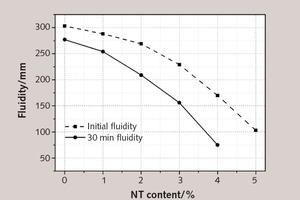
The various dosages of NT on the initial and 30 min fluidities of fresh SAC pastes are shown in Figure 7.
Figure 7 illustrates that the fluidity of fresh paste shows a diminishing trend for increasing addition of NT, because the cohesion among particles in the paste becomes stronger and the content of free water recedes [21]. The addition of 1 %, 2 %, 3 %, 4 % and 5 % NT makes the initial fluidity of fresh paste decrease by 4.95 %, 11.22 %, 24.42 %, 43.89 % and 66.01 %, respectively, due mainly to the fact that more free water or chemical admixtures are needed to achieve excellent performance in operation because of the larger specific surface area of NT. When the water binder ratio and super plasticizer content are fixed, NT with larger specific surface area can reduce both the volume between particles and the free water content, thus increasing the friction between solid particles and weakening the fluidity.
Moreover, with increasing dosage of NT, the 30 min fluidity of fresh paste shows the same varying tendency compared with the initial fluidity, and the fluidity loss with time gradually increases. When the NT content exceeds 3 %, the fluidity loss with time becomes obvious, with unfavorable effects on construction. For example, the addition of 3 % NT makes the 30 min fluidity decrease by 55.88 % compared to the initial fluidity. When the NT content reaches 5 %, the fresh paste shows no 30 min fluidity at all.
3.3 Setting time
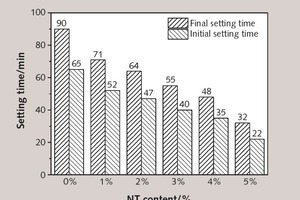
Nano-material has a heterogeneous nucleation effect in the process of SAC hydration, thereby modifying the hydration and hardening performance of SAC, which is one of the most important reasons for the results of NT use in cement-based materials. Figure 8 lists the setting time of SAC modified by different dosages of NT.
As Figure 8 shows, the initial and final setting times of SAC paste decrease along with increasing dosage of NT – specifically, the addition of 1 %, 2 %, 3 %, 4 % and 5 % NT reduce the initial setting time by 20.00 %, 27.69 %, 38.46 %, 46.15 % and 66.15 % respectively, and decrease the final setting time by 21.11 %, 28.89 %, 38.89 %, 46.67 % and 64.44 % respectively. One explanation for this could be that far smaller grains of NT substituting the same amount of SAC will cause a relative growth in solid volume and reduce the distance between particles of the composition cementing material system, hence promoting the formation of a net structure in the hardened cement paste and making the setting time shorter, while demonstrating that the addition of NT causes deterioration of the rheological and flow properties.
3.4 Pore structure analysis
Cement paste is a multi-component blend system, which is formed, developed and perfected gradually and includes crystals, gels, liquid and pores. Porosity is an important part of microstructure and has a major impact on the macro-performance of hardened paste. Therefore, the study of interior pore structure is significant for the analysis of paste performance. Laboratory tests by the MIP method of the influence of NT on pore structure of SAC at 8 h are listed in Table 4.
It can be seen from Table 4 that, with the addition of NT, the total pore volume, the average pore size and the porosity of the paste all decrease, while the apparent density gradually increases. For example, the addition of 1 %, 2 %, 3 %, 4 % and 5 % NT makes the total pore volume decrease by 16.33 %, 30.92 %, 37.86 %, 42.34 % and 48.12 % respectively, the average pore size decrease by 30.95 %, 41.10 %, 43.39 %, 47.68 % and 54.90 % respectively, the porosity decrease by 16.46 %, 20.25 %, 24.05 %, 31.65 % and 37.34 % respectively, and the apparent density increase by 0.75 %, 2.66 %, 5.81 %, 9.30 % and 13.29 % respectively. Simultaneously, it is also apparent that the addition of NT slightly shifts the pore size distribution in the direction of micro-porosity, i.e., NT can refine the pores of hardened paste, mainly because, when NT is incorporated, the high surface activity of nano-particles serving as nuclei in the paste can accelerate both the hydration of cement and the rapid formation of hydration products that gradually fill the pores and reduce the total pore volume [4]. Therefore, NT can make the microstructure of hardened paste become more compact and uniform, quickly form a mesh structure, and shorten the setting time.
3.5 Hydration heat
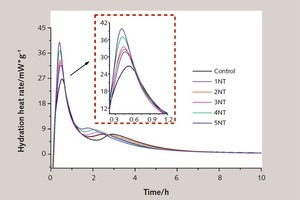
Cement hydration heat is the heat generated by the hydration reaction during the mixing phase of clinker and water. It has a major influence on the performance of cement, and the heat release rate reflects the early hydration process of cement to a certain extent. The hydration exothermic rate curves of pure and NT modified SAC are presented in Figure 9.
As shown in Figure 9, the first exothermic peak, which corresponds to the reaction of C3A, can be observed before 1 hour for all samples. This peak is followed by a secondary peak corresponding to β-C2S hydration. Also, the addition of NT increases the intensity of the first and second exothermic peaks and makes both peaks appear more quickly, Specifically, the addition of 1 %, 2 %, 3 %, 4 % and 5 % NT accelerates the appearance of C3A hydration peaks ahead by 12.50 %, 15.62 %, 18.75 %, 21.87 % and 25.00 % respectively, while the peak values increase by 18.63 %, 21.94 %, 25.37 %, 38.18 % and 48.89 %, respectively. At the same time, the appearance of β-C2S hydration peaks is accelerated by 15.91 %, 11.93 %, 24.43 %, 30.11 % and 40.34 %, respectively, while the peak values increase by 0.77 %, 0.72 %, 9.6 %, 22.6 % and 31.02 %, respectively, compared to the control sample. Prior studies have suggested that inert or active ultrafine particles evidently can promote cement hydration, which suggests that the primary role of the particles is to provide potential heterogeneous nucleation sites for hydration products and that the grain boundary region is densely populated with nuclei [22].
Based on the results of the hydration heat tests, it is suggested that NT particles can accelerate the reaction rate during the early hydration period and promote the formation and precipitation of hydration products. Accordingly, NT incorporation can decrease the porosity and considerably shorten the setting time. These findings may also explain why the addition of NT causes the rheological properties and fluidity of SAC paste to deteriorate.
3.6 XRD analysis of the hydrated cement pastes
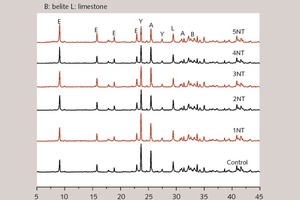
In order to discuss the influence of NT on the hydration products and microstructure of SAC at early ages, XRD patterns of different cement pastes at 8 hours have been outlined in Figure 10.
As Figure 10 shows, all samples produce ettringite (AFt, d: 0.972, 0.560 and 0.378 nm) with the continuous consumption of C4A3S and anhydrite and the addition of NT does not make the SAC system generate new hydration products. The reason may be that, when NT substitutes the same quantity of SAC, the average size of solid particle decreases and there are a lot of uneven atomic steps in the system, thus increasing the chemical reaction area [23]. In addition, at early ages, NT may provide many nucleation sites for the formation of hydration products, being favorable for the crystal growth of AFt [4]. Moreover, because the specific surface area of NT (50 m2/g) is much larger than that of SAC (0.41 m2/g), the effective water of SAC paste is lowered and the concentration of Ca2+, OH-, AlO2-, SO42- in liquid phase increased when NT substitutes an equal amount of SAC. This is conducive to AFt crystallization. At the same time, the smaller spaces between solid particles help the AFt form a network structure more easily [24].
3.7 Thermal analysis of the hydrated cement pastes
The DSC-TG analyses of different SAC pastes at 8 hours are shown in Figure 11.
There were three major endothermic peaks in all DSC curves. The first endothermic peak, relating to the bound water of AFt, occurred mainly within the range of 150 to 160 °C. The second endothermic peak, relating to the bound water of Al(OH)3 (AH3), occurred mainly within the range of 260 to 270 °C. However, no AH3 was detected in the XRD patterns, most likely because AH3 either crystallizes poorly or is in an amorphous form. The third endothermic peak, relating to the decomposition of CaCO3, occurred mainly within the range of 720 to 740 °C. Also, there is one-to-one correspondence between a decalescence peak in the DSC curves and a weight loss in the TG curves. At 8 hours, the first endothermic peak of samples with 3 % and 5 % NT is higher compared to the control sample. Hence, the weight loss also increases significantly compared to the control sample, indicating that the addition of NT is advantageous for the precipitation of crystallization. This is because NT acted as potential nucleation sites for the accumulation of hydration products [25]. All of this also confirmed the XRD results.
3.8 Hydration model
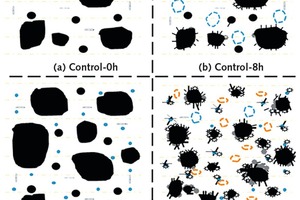
The hydration and hardening processes of sulpho-aluminate cement, though developed only a few decades ago, are already well understood. When exposed to water, the main SAC clinker of C4A3S reacts quickly with gypsum to form AFt and alumina gel. Then, the anhydrite content exerts major influence on the composition of the hydration products, especially those of AFt and AFm. When the anhydrite content is abundant, alumina gel and CH are generated earlier. Anhydrite and water continuously react to form AFt. When the anhydrite content is insufficient, AFm will be formed. Then, a small amount of C2S in SAC hydrates to form C-S-H gel and CH.
Based on the above experimental results, the hydration model of SAC can be built, as shown in Figure 12. At 8 hours, abundant AFt and alumina gels are generated in both the control sample and the NT-modified sample, accompanied by continuous consumption of C4A3S and anhydrite. At that point in time, many large pores are present in the control sample. However, the addition of NT increases the amount of AFt and gels in hardened paste, indicating that NT can promote the hydration of SAC. In addition, comparing Figure 12 (b) with Figure 12 (d), it can be seen that the pores are refined by NT incorporation. Therefore, the addition of NT makes the microstructure of hardened paste become more compact and uniform and some large pores start to transform into small pores. This model further evidences the influence of NT on the rheological properties, fluidity and setting time of SAC.
4 Conclusion
The findings of this research, as based on the experimental results obtained, are as follows:
The apparent viscosity and shear stress of fresh SAC paste increase when NT is introduced into the cement-based materials. This is more pronounced when higher amounts of NT are incorporated
The addition of NT makes the initial and 30 min fluidity decrease, and the extent of reduction and the time loss both increase with increasing NT content
The addition of NT shortens the initial and final setting time of SAC, and 1 %, 2 %, 3 %, 4 % and 5 % NT dosages make the initial setting time decrease by 20.0 %, 27.69 %, 38.46 %, 46.15 % and 66.15 %, respectively, and the final setting time decrease by 21.11 %, 28.89 %, 38.89 %, 46.67 % and 64.44 %, respectively
The pore size distribution of hardened paste is improved by NT. At 8 hours, the total pore volume, average pore size and porosity decrease, and the apparent density increases along with the addition of NT. Also, NT can refine the pores of hardened paste and slightly move the pore size distribution in the direction of micro-porosity
The addition of NT accelerates the appearance of a hydration peak and increases the hydration exothermic rate, indicating that NT could promote early hydration and accelerate the formation and precipitation of hydration products
The addition of NT significantly increases the generated quantity of AFt at 8 hours, and the rising degree gradually increases with additional NT. However, the types of hydration products generated do not change with the dosage of NT
5 Acknowledgement
The authors would like to acknowledge the 13th Five-Year Plan of National Key Research and Development (2016YFC0701003-05), the Science and Technology Support Program of Hubei Province (2015BAA084) and the Open Fund of State Key Laboratory of Solid waste recycling and energy saving building materials (SWR-2016-002) for supporting this research and providing the materials tested.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.