Investigations of the hydration of calcium sulfate hemihydrate
The application of calcium sulphate based binders need different properties, which are influenced by the morphology of the dihydrate crystals, and which depend on applied additives, reaction temperature or the production conditions of the reactant hemihydrate. The results show first only hemihydrate which is dissolved and the retarding period starts. Seed crystals of dihydrate are formed subsequently during the next few minutes.
1 Introduction
Gypsum is one of the most used materials in the world; 119 million tons of natural gypsum were extracted worldwide in 2006 (MINERAL COMMODITY 2007). Additionally another 200 million tons of byproduct gypsum came from different processes like phosphoric acid, citric acid or hydrogen fluorine production or from flue gas desulfurization.
Calcium sulphate based binders and gypsum are used for very different technical applications like floor pavement, stucco or plaster board in the building industry, for casting in the ceramic industry, as an additive in polymers or in colors or for...
1 Introduction
Gypsum is one of the most used materials in the world; 119 million tons of natural gypsum were extracted worldwide in 2006 (MINERAL COMMODITY 2007). Additionally another 200 million tons of byproduct gypsum came from different processes like phosphoric acid, citric acid or hydrogen fluorine production or from flue gas desulfurization.
Calcium sulphate based binders and gypsum are used for very different technical applications like floor pavement, stucco or plaster board in the building industry, for casting in the ceramic industry, as an additive in polymers or in colors or for plaster bondage or dental casting material in the medical sector and also as a food additive, either for humans (e.g. in ketchup or tofu), or in animal food. The principle customer is the cement industry where calcium sulphates are used to retard the very fast reaction from the clinker phase aluminate C3 A which consumes 51 % of the gypsum and for calcium sulphate based binders in building industry where another 39 % were used (Roskill 2004).
In this paper it will be shown how microscopy methods correspond to other analytical techniques and what a useful source of information they are about the phase transformations in the system of calcium sulphate hydrates with water. With optical microscopy it is possible to obtain a film of the hydration of hemihydrate with and without additives. Unfortunately it is not possible to show an example here, but there is one placed on the homepage of the Institute of Building and Materials Chemistry at the University of Siegen as a free download //www.chemie-biologie.uni-siegen.de/bwc/personen/pritzel.html" target="_blank" >www.chemie-biologie.uni-siegen.de/bwc/personen/pritzel.html:http://www.chemie-biologie.uni-siegen.de/bwc/personen/pritzel.html or use the qr-code on the last page of this article.
Another question is which phenomena occur during the reaction from calcium sulphate hemihydrate with water to calcium sulphate dihydrate:
2 CaSO4 ∙ 0.5 H2O +3 H2O -> 2 CaSO4 ∙ 2H2O
In the literature different theories are considered. The growing of gypsum crystals out of a supersaturated liquid was described by Lavoisier and Le Chatelier in 1919 [2]. Cavazzi and Traube described a gel like interface and a stepwise transformation into a crystal form of dihydrate.
Another kind of reaction was published by Fiedler (1958). He described the crystallization of gypsum as an inner reaction in the calcium sulphate subhydrates by an inner transformation of the crystal structure.
Perederji in 1956 [1] and Eipeltauer in 1960 combined the theories of Lavoisier and Le Chatelier and Fiedler and published that there will be an inner hydration beside the creation of dihydrate out of a super-saturated solution.
2 Results and discussion
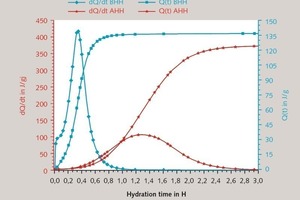
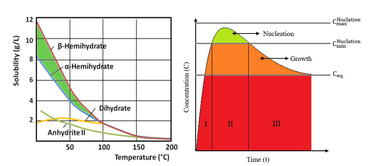
In a first step the hydration of hemihydrate was observed by heat flow calorimetry. The calorimetric investigation has shown the heat evaluation and the progress of the hydration. Figure 1 shows the results of heat flow calorimetrical investigations from α-hemihydrate (α-HH) und β-hemihydrate (β-HH).
Four reaction steps can be identified. The first step is the initial period, where hemihydrate becomes dissolved. During the second period seeds were created and start to grow, normally that is the only step where seed crystal formation takes place. The third reaction period is the main reaction period. In this period the equilibrium of dissolution of hemihydrate and of crystal growth is reached. When the heat gets lower most hemihydrate is solved, the amount of ions which are needed for the crystal growth cannot be delivered by dissolving of hemihydrate. This is the retarding period. For the microscopic examination of the selected four characteristic times according to the temporal change of the heat flow:
at the beginning,
beginning of the increase,
at the maximum, and
at the end of the reaction.
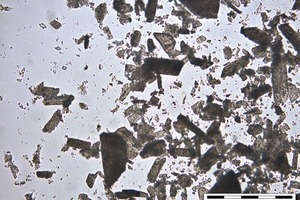
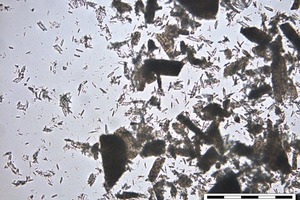
A special measuring cell for in situ optical microscope measurements was created. The cell was constructed from a rubber ring which is glued with water insoluble grease on a slide, filled with hemihydrate and water and closed with a cover glass which is also glued with grease. After starting the reaction by adding water a picture is taken under the optical microscope every thirty seconds, the first one after about 20 to 40 seconds. The results of the investigation of hemihydrate hydration by OM are given in Figures 2-13.
For the seed crystal creation the ion concentration has to be above the Oswald-Miers-Range. If it is in this range crystals are only growing and no formation of seed crystals takes place. This concentration is only reached in the beginning of the reaction; afterwards the created seed crystals are growing and catching ions out of the solution so that the ion concentration in the solution becomes lower. If the ion concentration in the solution gets much higher than the Ostwald-Miers-Range more seeds are created and the dihydrate crystals at the end of the reaction are smaller (like β-HH). If the concentration gets a bit lower, less seeds are created and the dihydrate crystals get larger (like α-HH). More expansion during hydration and more porosity can be expected.
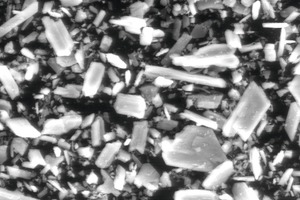
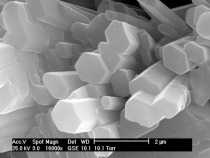
The solubility and the dissolution rate of α- and β-HH are different, which depends on the particle size and the amount of defects on the crystal surfaces. By having a look at α- and β-hemihydrate by the SEM (Fig. 10, Fig. 14) this fact is proven clearly. Examples of these pictures are shown below, α-hemihydrate with large crystals and less visible defects and β-hemihydrate with smaller crystals. In addition a picture is shown where a dihydrate crystal was dehydrated, which created cracks along the c-axis.
Another investigation was ESEM measurement of the hydration of hemihydrate. For the ESEM investigations the samples were mixed in sealed sample vessels with water. At the times, which according to the heat flow calorimetrical results had been determined (same times like by OM investigating), they were sampled and examined. Before the sample was stored in the SEM, the reaction solution was removed from the sample. After the sample was stored in the SEM, pictures were taken in ESEM mode. The normal reaction time could be measured and no crystals of different generations were found. The results of the investigation of hemihydrate hydration by ESEM are given in Figures 14-17.
In addition to the given pictures others were taken after shorter times. By that it is possible to detect even very small crystals which could not be detected with optical microscopy. With ESEM, no real in situ measurement is possible, because the water has to be evaporated before pictures could be taken. The disadvantage of the optical microscopy is that very high water gypsum ratios are needed. Because of that it is necessary to check the results using another method. To check the crystal morphology and the reaction progress at special chosen times during the hydration reaction ESEM could be used. To investigate the hydration of hemihydrate optical microscopy and ESEM are working together perfectly. Optical microscopy could do the in situ measurements and ESEM is needed to prove the results and to have a closer look at the first minutes of the reaction with a higher resolution.
3 Conclusion
It was shown that three phenomena take place during the reaction of hemihydrate with water. Hemihydrate becomes solved to create a supersaturated solution, where dehydrate crystals start to fall out and grow beside an inner hydration. The seed crystal creation was detected only in the first seconds and influences the crystal size, morphology and technical properties of the product dihydrate. To any step which could be found with heat flow calorimetry the phenomena which are taking place could be explained. Primarily hemihydrate is dissolved in water and then seed crystals are formed. During the main reaction period dissolution of hemihydrate and growth of dihydrate reaches equilibrium and at the retardation period more calcium and sulphate ions are necessary for crystal growth which could not be delivered by dissolution of remaining hemihydrate. The optical microscopy could be used to follow the hydration process in situ in water. By polarization light microscopy it is possible to obtain additional information like twinning of crystals for example. So this cheap method with an easy sample preparation leads to good information about the hydration process, but the resolution is limited by the wavelength of visible light. To increase that resolution the ESEM technique could be used but the ESEM technique has to be used very carefully and with sample preparation outside of the ESEM.
ESEM has several advantages in comparison with a normal SEM. For SEM, the non-conductive samples must be made conductive by using a sputtering medium. With SEM in the sample chamber exists a high vacuum. ESEM does not need a sputtering medium and no high vacuum in the sample chamber. The high vacuum may be a problem for gypsum samples, because in the samples may arise cracks and artifacts by the drying due to the high vacuum. With ESEM it is not possible to look through the water, so the sample preparation gets more difficult, because the water has to be removed for any picture. The advantage of this technique is the high resolution and the fast measurement.
The results had shown that the ESEM technic is very useful and enables to consider the hydration process and the hydration products with a new perspective.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.