Carbonate CO2 scrubbing process II (ECO2) – Practical experience and technical optimisation
This article describes the practical experience and optimisation of the carbonate CO2scrubbing process at the Uniper coal-fired power station in Wilhelmshaven. It focusses on CO2 capture and the development of a buffering function. Since the CO2 scrubbing process can in principle be applied to almost all CO2 point sources, it can be used across industrial sectors and applications for climate protection and CO2 reduction.
The flue gas scrubbing was researched as part of the AiF IGF research project (IGF No. 18560 N, [1]). The project includes the optimisation of CO2 removal from the flue gas with simultaneous formation of a mineralised water, which is to be used directly for water body remediation and buffering. In this process, the natural limestone-weathering cycle is mimicked and artificially accelerated within the mobile demonstration plant. The advantage of the process is the long-term conversion of limestone powder, water and CO2 into calcium bicarbonate in dissolved form, which can be used as a pH...
The flue gas scrubbing was researched as part of the AiF IGF research project (IGF No. 18560 N, [1]). The project includes the optimisation of CO2 removal from the flue gas with simultaneous formation of a mineralised water, which is to be used directly for water body remediation and buffering. In this process, the natural limestone-weathering cycle is mimicked and artificially accelerated within the mobile demonstration plant. The advantage of the process is the long-term conversion of limestone powder, water and CO2 into calcium bicarbonate in dissolved form, which can be used as a pH buffer. This article focusses on the CO2 capture and buffer development. During the project the effects of discharging the mineralised water on the marine carbonate system were also modelled, and the water chemistry during the limestone meal CO2 scrubbing was analysed in order to estimate the environmental impact on aquatic flora and fauna [1,2]. In principle, the CO2 scrubbing process can be applied to almost all CO2 point sources and can therefore be utilised across all industries and applications for the purpose of climate protection and CO2 reduction.
1 Introduction and fundamentals
Slowing down the climate change is one of the most important tasks and challenges of our time. To achieve this aim it is of extraordinary importance to reduce greenhouse gas emissions, especially carbon dioxide (CO2). The introduction of an emissions trading scheme in the EU, involving a steadily decreasing emissions budget, has created an incentive to reduce CO2 emissions. For this purpose, various technologies for the separation of CO2 from the flue gas of combustion processes have in the past already been researched and tested experimentally and in pilot plants. All of these so-called post-combustion processes have in common that they generally use environmentally critical substances to achieve the separation, in some cases producing new emissions and, as elements of the CCS concept (Carbon Capture and Storage), involve the problematic storage of the separated CO2 in underground reservoirs.
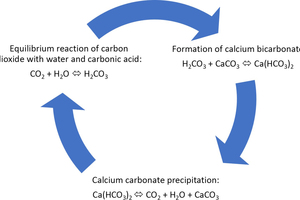
The process of carbonate CO2 scrubbing, the fundamentals of which had already been developed in an AiF predecessor project [3], pursues a holistic approach that offers the chance of broad sociopolitical acceptance. In this process, the CO2 is converted into water-soluble calcium hydrogen carbonate (so-called carbonate hardness) by reaction with limestone meal or chalk. For this purpose, flue gases containing CO2 are subjected to wet separation employing limestone meal or chalk suspension similar to the flue gas desulphurisation process. Instead of calcium sulphate, calcium bicarbonate is formed in a similar manner to the natural weathering of limestone (Figure 1). Calcium bicarbonate is a natural component of limnic and marine aquatic systems and binds CO2 into an aqueous solution in a stable lime-carbonic acid equilibrium.
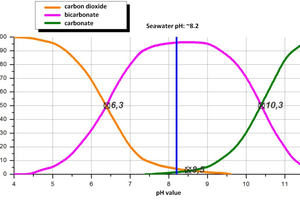
The chemical basis of these processes is the carbonate balance. This describes the chemical equilibrium between the different carbon compounds carbon dioxide (CO2), bicarbonate (HCO3-) and carbonate (CO32-), depending on the pH value, and determines the lime-precipitation or dissolving character of the water (Figure 2). In natural water bodies (ocean, lake, river, groundwater) the pH value is determined by the content of dissolved CO2 and free carbonic acid (H2CO3) in the water, as the system is in equilibrium with the respective CO2 content in the atmosphere. Carbon dioxide dominates at pH values below 6.3, changing into bicarbonate as the pH increases and being replaced by carbonate as the predominant carbon compound as from a pH of 10.3. This equilibrium determines the buffering capacity of the water [4]. The alkaline action of the alkaline earth ion calcium in natural waters ensures that a stable pH environment (pH 6.3 - 8.3) exists around the neutral point during the dissolving or precipitation of calcium carbonate. Excesses of free carbonic acid are converted from limestone deposits into Ca(HCO3)2.
In a theoretical approach, the carbonate CO2 scrubbing process has already been calculated with regard to potential costs and water volumes required by experts including Ken Caldeira, geoengineering specialist at Stanford University and one of the IPCC lead authors [5, 6, 7]. These authors consider the process to be usable for power plants located near the sea (cooling with sea water), due to the required high amounts of water.
2 Objective
The objective of the research project was, on the one hand, the economic and technical optimisation of the process of carbonate CO2 scrubbing to a state of maturity and, on the other hand, the development of a model to verify the expected ecological effects based on the chemical analysis of the process water and knowledge of the natural aquatic environment. For this purpose, a mobile demonstration plant was designed and built for processing up to 200 m³/h of flue gas, serving to prove both the suitability for practical use and the cost-effectiveness of the process.
Implementation of the carbonate CO2 scrubbing process would bring about a new approach to a CO2 reduction strategy that does not rely on the storage of excess CO2, but seeks to develop a process that converts CO2 into naturally occurring, environmentally neutral substances (carbonate hardness) which, in the form of water with a strong buffering capability, can be returned to the natural carbon cycle or utilised in subsequent production processes. Aside from the positive aspect of avoiding the classical CO2 capture and storage, there is a reasonable hope that the new carbonate CO2 scubbing process, in addition to reducing CO2 in flue gases, is suitable for counteracting the acidification of water bodies under certain circumstances.
Another advantage of the new process would be that – in contrast to other CCS technologies – the carbonate CO2 scrubbing process does not make use of environmentally hazardous substances. The simple and energetically favourable nature of the process is also a positive characteristic. In contrast to CCS processes in which the carbon dioxide absorbed by the scrubbing medium in the absorber is separated again in the desorber by supplying large amounts of heat energy, the CO2 remains stable primarily in the form of bicarbonate in the mineralised water after the carbonate scrubbing and becomes a natural constituent of the water discharged back into the water body used for supplying the process. For the scrubbing process, only water, limestone powder or chalk and pump energy are required. There is no need for the energy consumed by other processes for injecting the CO2 into the subterranean strata.
3 Methods and practical testing
The design of the demonstration plant for flue gas scrubbing with limestone is based on experience gained in the previous project [3] for demonstrating the general suitability of the process concept of carbonate CO2 scrubbing using a simple adsorption system. This database was complemented by kinetic studies using sea water carried out on a laboratory-scale with a modified loop reactor to determine the sorption and dissolution kinetics of the limestone meal-water-CO2 system. The specific site conditions were also taken into account for the design and scaling of the CO2 scrubber.
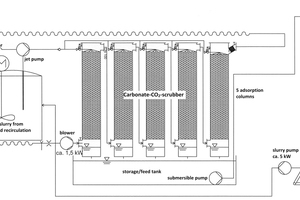
The scrubber (Figure 3) was designed as a five-stage cascade mounted on a pump storage/feed tank. The five gas scrubbing columns (length 1950 mm, diameter 315 mm) were made of polypropylene. They each contained an internal sieve bottom supporting a packed bed 1500 mm in hight. Installed above each packed bed is the distribution piping for the 6 m3/h throughput of scrubbing water at 1.5 bar water pressure. The scrubber columns are interconnected with plastic pipes and the inlet. The inlet at the bottom of each column is connected to the outlet of the predecessor column. The flue gas passes from bottom to top through the scrubber continuously mingling with the scrubbing water coming from above. Due to the strong turbulence and the available surface of the packed bed, an intensive mass transfer takes place, promoting the conversion of carbon dioxide into calcium bicarbonate within the CaCO3-CO2-H2O system.
The carbonate sea water suspension is produced in a separate mixing station consisting of two water jet pumps, each of which convey up to 20 m3/h of operating water. A ~ 35% chalk suspension is utilised as the suction medium, which is also used in the flue gas desulphurisation (FGD) plant of the power plant. It is provided in modified IBC containers, each equipped with an agitator and a suction lance. The produced scrubbing suspension is sprayed from above onto the packed beds, trickles through them and passes through the sieve bottom to the outlet of the columns and thus into the pump storage/feed tank. From there, the used scrubbing water is conveyed by means of a submersible pump to the so-called Dortmund tank. This is a hopper with a diameter of 3000 mm and a height of 4200 mm and facilitates rapid sedimentation of the limestone meal. The sea water containing the calcium bicarbonate is discharged via a clear phase overflow. The lime slurry discharged from the hopper outlet of the Dortmund tank is conveyed by a peristaltic pump to the scrubber feed tank and recycled.
The field trials carried out at the Wilhelmshaven coal-fired power plant spanned a period from spring 2017 to summer 2018.
4 Results
The evaluations of the individual test campaigns (see test number, Table 1) are carried out from a point in time at which a constant volume flow (of flue gas and suspension) has been established in the system. It can then be assumed that a quasi-stationary state exists, allowing the amount of removed CO2 to be calculated. During all test campaigns, the proportion of CO2 in the flue gas was between 7.6 % (test number 1) and 12.6 % (test numbers 3 and 4), with an average value of 11.1 %. The concentrations of CO2 in the clean gas varied between 2.3 % (test number 1) and 9.6 % (test number 12), indicating a CO2 removal of 17 to 70 %. The pH value of the mineralised water varied between pH 6.8 and pH 8.2, while the initial pH of the supplied sea water was 8.2. The alkalinity as an indicator of the increase in the buffering capacity rose significantly in the course of the test campaigns from an initial value of ~ 2.3 to values of between 2.5 and 4.8. All other measured parameters can be found in Table 1. The calculations are in Berry et al. (2018) [1].
5 Discussion
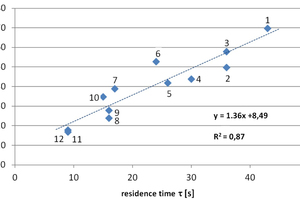
Figure 4 illustrates the results of the practical tests using flue gas from the chimney of the power plant, also listed in Table 1. In this diagram, the average percentage of CO2 removed from the flue gas by the carbonate CO2 scrubbing in the respective tests is plotted against the residence time. The residence time quantifies the time in seconds that a flue gas molecule takes to flow through the scrubber from its entrance to its exit.
Despite some deviations in the individual test parameters which are unavoidable in field operation, such as the sea water temperature and the ambient temperature (summer and winter operation), the load-dependent CO2 content in the flue gas, the flue gas temperature, the chalk content in the suspension etc., it is nevertheless clear that the CO2 removal performance is a function of the residence time. With residence times over approx. 24 s (corresponding to the experiment numbers 1 to 7 and flue gas flow rates of 42 to 109 Nm3/h), the achieved CO2 reductions were in the range of 40 to 60 %, and in individual cases even reached 70 %. Residence times below approx. 24 s down to 9 s (corresponding to a maximum tested flue gas volume flow of 200 Nm3/h, test numbers 8 to 12) lead to a lower CO2 removal performance between 17 and 35 %. By optimising the CO2 scrubber in relation to the optimal ratio of volume flow to residence time, it will therefore be possible to achieve relevant and consistently high removal rates with the carbonate scrubbing process.
Furthermore, besides the residence time, the temperature of the scrubbing suspension also represents a parameter relevant to the CO2 removal efficiency. All data points above the trend line in Figure 4 relate to tests carried out in spring, where the ambient and thus also the scrubbing bed temperatures were approx. 5 - 11°C, i.e. well below the temperatures prevailing during the summer operation. At lower temperatures, CO2 is generally more soluble in water. For practical operation of the process, it is therefore advisable to obtain the water required for generating the scrubbing suspension from deeper, colder water bodies as well as to discharge the mineralized water at greater depth.
6 Summary
In summary, the following findings were made:
The degree of CO2 removal was 40 to 60 %/70 %, depending on the flow rate of the flue gas
Scrubber optimisation might enable consistently high CO2 removal rates, depending on the ratio of residence time to volume flow and CO2 concentration in the flue gas
The relationship between CO2 removal and development of the buffering capacity of the mineralised water can be adjusted individually according to local needs
Depending on the availability of water, CO2 limestone meal scrubbing can in principle be used at all CO2 point sources as an end-of-pipe solution
The desired increase in the bicarbonate buffering capability was proven
There was no undesirable accumulation of main or trace elements in the mineralised water [1]
One-year simulations show that ~ 50 % of the removed CO2 remains bound in the mineralised water, even if it is discharged near the surface of the water body [1, 2]
Long-term binding of all the removed CO2 is made possible through a discharge point away from the coast in deeper water regions. The advantages: dilution effects and pressure differences
CO2 removal costs are ~ 250 €/t of CO2 for the pilot plant. For an industrial plant they would be considerably lower. There are economic advantages compared to MEA scrubbing [1]
7 Prospects
Both, the positive results of the CO2 sequestration and the buffering efficiency of the mineralised water are to be utilised as part of a follow-up project for lake remediation in Lusatia/Germany. The aim of this project is to initially neutralise an acidified open pit mine lake with quicklime and limestone meal and then to carry out a continuous operation of the limestone meal CO2 scrubbing system in circulation mode. This would provide a sustainable solution for remediation of the lake water which, at the same time would bind CO2 in a manner that would benefit the climate.
Acknowledgements
The research project was funded by the German Federal Ministry for Economic Affairs and Energy (BMWi) within the framework of the programme for promoting cooperative industrial research (IGF-No.18560 N) through the German Federation of Industrial Research Associations (Arbeitsgemeinschaft industrieller Forschungsvereinigungen AiF e. V.) on the basis of a resolution of the German Bundestag. Special thanks also go to Uniper Kraftwerke GmbH, in particular to the employees of the Uniper Kraftwerk Wilhelmshaven for their active support of the on-site field tests.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.