An alternative material to increase the photocatalytic performance of TiO2
The Adana Cement R&D group is working on a material that is able to boost photocatalytic performance of cement, yet costs less than TiO2. This material was named the “TiPlus”. Use of TiPlus not only increases performance, but also replaces TiO2 to a large extent and is able to reduce production cost by 21-37 % depending on the preferred level of performance. It is thought that photocatalytic cement will be used more widely in the industry thanks to this reduction in its production cost.
1 Introduction
It is nowadays possible to maintain concrete surfaces clean and spotless for years. Concrete structures with such clean surfaces can be obtained by using properties of self-cleaning cement, which is able to break down organic dirt that piles up on concrete surfaces and keep the surface clean by displaying photocatalytic features under sunlight. In the meantime, it is able to photocatalytically convert NOx compounds into harmless compounds, drive them away and reduce NOx concentrations present in the air, thanks to the nano-sized TiO2 particles in its content. Self-cleaning cement...
1 Introduction
It is nowadays possible to maintain concrete surfaces clean and spotless for years. Concrete structures with such clean surfaces can be obtained by using properties of self-cleaning cement, which is able to break down organic dirt that piles up on concrete surfaces and keep the surface clean by displaying photocatalytic features under sunlight. In the meantime, it is able to photocatalytically convert NOx compounds into harmless compounds, drive them away and reduce NOx concentrations present in the air, thanks to the nano-sized TiO2 particles in its content. Self-cleaning cement thus enables clean surfaces which do not accumulate dirt, while reducing NOx emissions induced by intensive urban traffic. NOx limits which have recently been introduced as part of various European Commission directives have promoted both use of and research on these products. However, use of self-cleaning photocatalytic cement is not yet at the anticipated levels due to the high costs associated with the TiO2 content.
Researchers started focusing on the photocatalytic properties of TiO2 around the late 1960’s [1]. Applications where water was photocatalytically split into its constituents using a TiO2 anode, in particular, led researchers to focus on its photochemical oxidative reactions [2]. Recent research has been conducted on photocatalysis processes such as photocatalytic cleaning of water and air [3], self-cleaning [4] and antibacterial surfaces [5]. TiO2, a semi-conductive, is used in paint, cosmetic products and foodstuff as a white pigment. It has three crystalline forms, namely anastase, brookite and rutile. Of the three forms, anastase displays the highest level of photocatalytic activity. In addition to the ability of cleaning itself, self-cleaning concrete surfaces convert the NOx gases present in the air into the harmless HNO3 compound. This makes it possible to neutralize NOx compounds caused by exhaust fumes especially in metropolitan areas, creating healthier and cleaner spaces. In addition, the European Council directive 1999/30/EC, which is applicable in European Union member states, has introduced limits for NOx concentrations in urban areas. Despite the introduced limits, the number of vehicles with diesel engines, which increase traffic-induced NOx emissions, keeps going up [2]. Owing to this increase in NOx in urban air and the limits laid out in the directive, use of photocatalytic concrete is expected to become widespread for the purpose of NOx reduction.
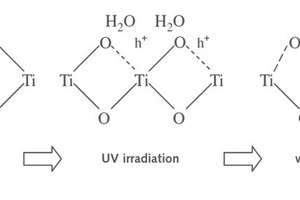
2 Self-cleaning mechanism
The self-cleaning process takes place as a result of two primary photochemical events occurring on the photocatalytic surface under ultraviolet light:
Redox reactions of compounds adsorbed on the surface; and
The surface gaining super hydrophilic properties.
These two events form the basis of photocatalytic applications in the construction industry [2]. The principle underlying semi-conductive photocatalytic reaction is simple. Semi-conductive materials have one full valence band and one empty conductivity band. Bands are the probable energy levels in a material which can be occupied by an electron. Electrons on the external orbit are named ‘valence electrons’. Valence electrons are responsible for forming bonds between atoms. The band at the highest energy level which has valence electrons on it is called the valence band (VB). The semi-conductive is irradiated with light containing higher energy than the band interval. With this energy, the conductivity band (CB) is split and the valence band is filled. The electron is then induced to be sent toward the CB. The reaction thus results in the pair of an irradiated electron (e-) and a positive hole (h+). These e- and h+ oxidize and reduce chemical substances present on the surface of the photocatalyst. If an equal number of e- and h+ has been consumed during the chemical reaction/recombination, the original chemical composition of the semi-conductive material remains unchanged. The term ‘photocatalysis’ stems from this event [6].
2.1 Redox reactions of compounds adsorbed
on the surface
2.1.1 Breakdown of organic components
Below is the breakdown on palmitic acid as an example describing the phases of breakdown of an organic component on a photocatalytic concrete surface and the resulting components:
TiO2 is irradiated with ultraviolet light:
TiO2 ➝ h+ + e-
n-C15H31 – COO-H+ + h+ ➝ n-C15H31 – COO· + H+
➝ n-C15H31· + CO2 + H+
It is then converted into alcohol through OH- oxidation:
n-C15H31· + OH· ➝ n-C15H31OH
Oxidation of the formed alcohol leads to formation of aldehyde and n-C14H29 – COOH. These reactions go on until the entire palmitic acid is converted into H2O and CO2 as a result of a chain of reactions.
2.1.2 Neutralization of NOx components
Conversion of the gases of NO and NO2 into HNO3 occurs in the following sequence:
TiO2 is irradiated with ultraviolet light:
TiO2 ➝ h+ + e-
h+ impacts the OH- ion and forms a hydroxil radical:
h+ + OH- ➝ OH·
e- impacts molecular oxygen and forms a superoxide anion:
e- + O2 ➝ O2-
A water radical is formed, which then converts NOx compounds into HNO3:
H+ + O2- ➝ HO2·
NO + HO2. ➝ NO2 + OH·
NO2 + OH· ➝ HNO3
2.2 Formation of super hydrophilic surface
When a surface containing TiO2 is irradiated under ultraviolet light, the angle of contact between water and the surface is reduced to an almost non-existent extent. On such a surface, water molecules tend not to form round drops. Instead, they spread along it. Due to this spreading motion, waste can be driven away from the surface by water as a result of the self-cleaning process.
The bonding energy between Ti and O wears out when TiO2 is irradiated under ultraviolet light. Therefore, water molecules clinging to the surface snap the Ti-O-Ti bond, replacing it with Ti-OH bonds and making it possible for a super hydrophilic surface to emerge.
The simultaneous occurrences of a cleaning impact on the surface and spreading of water on the entire surface through its super hydrophilic capacity make this a highly effective method for construction materials due to the self-cleaning effect [3].
3 Effect of TiPlus
Compounds with ferroelectric characteristics are used to enhance the photocatalytic performance of TiO2. Ferroelectric crystals such as LiNbO3 and BaTiO3 utilize UV light more efficiently. Ferroelectric optical materials can guide UV light into the catalyst interiors, enhance scattering, and stabilize the electron-hole pairs. The results of the studies indicate that ferroelectric crystals may enhance photocatalysis through the mechanisms such as refraction and transmission through the crystal or prolongation of lifetime of electron-hole pairs due to the ferroelectric effect. [7, 8].
Low-cost components with ferroelectric properties were used in TiPlus, which was developed at Adana Cement. The photocatalytic performance boost provided by TiPlus can be observed in the activity test results found below.
3.1 Determination of photocatalytic activity
In this study, photocatalytic activities of cement samples are tested according to the UNI 11259:2008 standard (“Determination of the photocatalytic activity of hydraulic binders”). This standard is also known as the rhodamine test. This colorimetric method allows monitoring of the photocatalytic activity of the cement over the course of time for a maximum of 26 hours under a UV-A lamp. The rhodamine B, which is a red organic dye, is used as a pigment and applied as a solution on the surface of the cement-based specimens (prepared according to EN196-1). Photocatalytic activity is measured with reference to rhodamine discoloration. The CIE L*a*b* colorimeter is used for the colorimetric measurement, measuring “a*”.
Prior to exposition to the UV-A lamp, an initial measurement of a* is performed, which is represented with a*(0). The lamp is then switched on and UV-A irradiation starts. Measurements are made after 4 hours [a* (4)] and after 26 hours [a* (26)].
R4 = [a*(0) – a* (4)] x 100
a*(0)
R26 = [a*(0) – a* (26)] x 100
a*(0)
If it is observed that R4>20 % and R26>50 %, the cement is considered to be photocatalytic [9].
3.1.1 Test results
When 10 % TiPlus is added to photocatalytic cements with various TiO2 contents, a considerable increase is observed in photocatalytic activity. Based on their TiO2 content, the samples were labeled as T-3, T-4 and T-5. The samples in which 10 % TiPlus was added were similarly labeled as TP3, TP4 and TP5, also based on their TiO2 contents (Table 1).
According to these results, the sample containing 3 % TiO2 (T-3) does not meet the standard. However, after adding 10 % TiPlus, TP3 becomes photocatalytic cement according to the R4 and R26 values. T-4 and T-5 also meet the standard, but photocatalytic activity of TP3 is higher than T-4 and T-5. The test results show that addition of TiPlus and decreasing the amount of TiO2 increased the photocatalytic property of the cement. In addition to this performance increase, the production cost of TP3 is 21 % and 37 % less than T-4 and T-5 respectively.
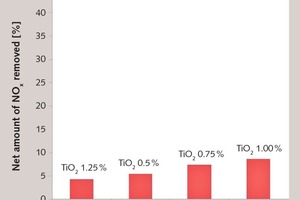
3.2 Determination of NOx removal capability
NOx removal capabilities of T-5, TP-2, TP-3 and TP-4 were determined by Queen’s University Belfast, IPS (International Photocatalyst Standards Testing Center). ISO 22197-1: “test methods for air-purification performance of semiconductor photocatalytic materials” standard is used for advanced technical fine ceramics but it satisfies the need for measurements on concrete specimens as well. The samples were prepared according to the EN 196-1 standard. The amount of TiO2 on the concrete surfaces were 1.25 %, 0.5 %, 0.75 % and 1 % respectively.
Although containing less TiO2, thanks to the accelerator effect of TiPlus on photocatalysis, the NOx removal capabilities of TP-2, TP-3 and TP-4 were higher than T-5.
4 Effect of TiPlus on physical properties
of the cement
As results of photocatalytic activity and NOx removal test, the sample of TP3, which present the highest production cost difference with comparable photocatalytic cement properties, is selected for testing physical properties.
According to the physical test results, the standard strength class of 52.5 N is obtained by using TiPlus. When analyzing these results attention should be paid to the fact that TiPlus replaces clinker and leads to a reduction of 10 % in cement clinker. The 2-day and 28-day strength performance is quite striking despite such a reduction in clinker ratio.
5 Conclusion
In conclusion, self-cleaning concrete containing photocatalytic cement will become more important in the near future in terms of both aesthetical and environmental concerns. Using photocatalytic nano-TiO2 in the form of anastase is the common way of producing photocatalytic cement. However, due to the high cost of TiO2, the use of photocatalytic cement is currently limited in the world construction industry.
According to the results obtained in this study, TiPlus, which was developed at Adana Cement, boosts the photocatalytic performance of TiO2 and allows addition of lower amounts of TiO2. The reduction in the amount of TiO2 can reduce the production cost of self-cleaning photocatalytic cement by up to 21-37 % depending on the preferred level of photocatalytic performance. Thanks to this cost advantage, it is thought that these products will gain a wider user base.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.

![1 Occurrence of semi-conductive photocatalytic reactions [3]](https://www.zkg-online.info/imgs/tok_30ec440502cfcf71cfeeb92125e332ce/w300_h200_x400_y310_101549598_ef25d25582.jpg)