Research progress of nitrogen oxides (NOx) control technology for industrial flue gas
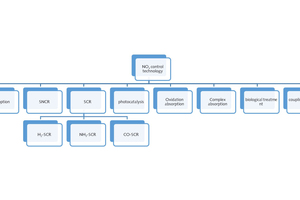
The paper reviews several denitration technologies, such as adsorption, reduction, photocatalytic, oxidation-absorption and biological denitration, etc. In order to improve denitration efficiency, it can also be coupled with UV, microwave and electrochemical technology. The denitration mechanisms are introduced and combined with the latest research progress of the report to make a specific description, a brief evaluation and comparison. Finally, the flue gas denitration technology is summarized and prospects presented, in order to provide reference for the progress of new denitration technology and the early industrial application.
1 Introduction
With the development of the economy and the progress of technology, energy consumption is growing fast. Along with the increasing of energy consumption, atmospheric pollutants are increasing. Nitrogen oxide, including NO, NO2 and N2O3 etc., is one of the main atmospheric pollutants, which causes harm to the ecological environment and human health, and also can form acid rain and photochemical smog. In China, with the gradual implementation of the ultra-low emission requirement, the state has set limits on the emission concentration and total emissions of nitrogen oxides.
China has...
1 Introduction
With the development of the economy and the progress of technology, energy consumption is growing fast. Along with the increasing of energy consumption, atmospheric pollutants are increasing. Nitrogen oxide, including NO, NO2 and N2O3 etc., is one of the main atmospheric pollutants, which causes harm to the ecological environment and human health, and also can form acid rain and photochemical smog. In China, with the gradual implementation of the ultra-low emission requirement, the state has set limits on the emission concentration and total emissions of nitrogen oxides.
China has promulgated and revised the emission standard of air pollutants many times. For example, the NOx emission limit decreased from 1600 mg/m3 (GB 4915) in 1996 to 800 mg/m3 in 2004, and then decreased to 400 mg/m3 in 2013 (320 mg/m3 in key areas) in the cement industry (Table 1). In view of the fact that the coal power industry has basically achieved ultra-low emissions (NOx ≤50 mg/m3), since 2018, Henan and Hebei province have formulated stricter emission standards and reduced the emission limit of NOx to 100 mg/m3 or even 50 mg/m3 in the cement industry. More strict national standards are also being formulated.
The main component of NOx is NO, and NO is poorly absorbed by the absorption liquid because it is difficult to be dissolved, no matter through dry or wet denitration technology. Only through physical adsorption, NOx decomposition or reprocessing after reduction/oxidation can achieve the purpose of denitration. At present, dry denitration technology mainly includes adsorption method, reduction method and oxidation method. Since NO is difficult to dissolve in the absorption liquid, resulting the low denitration efficiency. When use wet denitration method, NOx is usually oxidized to higher valence first, and then use different methods to fixed down the high valence NOx in the liquid phase to achieve the purpose of denitration. As can be seen from Figure 3, NOx emission from exhaust gases discharged by centralized pollution treatment facilities in China has gradually increased in recent years.
2 Adsorption
Adsorption technology refers to the use of modified activated carbon fiber/molecular sieve or other adsorption materials with large specific surface area and porous to directly adsorb NOx. Some of the adsorption materials can also oxidize NO to NO2. The adsorption products are recycled after follow-up treatment. The technology can realize desulfurization and denitration simultaneously because of the broad spectrum adsorption characteristics of the adsorption material.
The operation of adsorption technology to remove NOx is simple. It does not need to consume other substances, does not produce waste water and waste residue generation, and can remove some heavy metal pollutants in the flue gas at the same time. But the adsorption capacity of adsorption materials is limited, the amount of adsorbent and the process equipment is large, and also the investment cost is high. So the adsorption method is only suitable for denitration with low NOx concentration of flue gas. Moreover, this method is not a thorough NOx treatment method. The adsorbent is required to be regeneratied after frequent use, which can easily cause secondary pollution, so there are few industrial applications reported.
3 SNCR
Selective non-catalytic reduction (SNCR), which means NOx and some reductants (such as urea NH3 containing amino substances) react and generate pollution-free N2 and H2O under the condition of no catalyst and high temperature (generally 900~1100 °C), so as to achieve the purpose of denitration. SNCR has advantages such as low investment cost, a simple and economical denitration device and convenient operation. So SNCR technology has long been widely used.
The focuses of SNCR technology is to optimize the reaction parameters, improve denitration efficiency and reduce ammonia escape. The study of Wang [2] et al. indicates that adding trace CO to the SNCR reactor can make the optimum reaction temperature and denitration temperature window move towards lower temperature, which reduce from 800 °C to 750 °C, and denitration efficiency increased slightly. The ammonia escape curve also significantly shifts to the low temperature direction. The emissions of NO2 concentration is in the lower peak and moving in the direction of low temperature.
In order to test the application effect of SNCR denitration technology, Chen [3] et al. carried out an industrial experimental study of SNCR denitration technology in an 8.4 MW organic heat carrier boiler furnace. The industrial SNCR denitration test platform was built by using urea as reducing agent. The SNCR denitration experiment was carried out with urea solution of 10 %, 15 % and 20 % concentration under different urea injection volume, different oxygen content and different boiler load. With the increase of urea injection and the reduction of boiler load, the NOx concentration at the outlet decreases, and the denitration efficiency increases. Under different oxygen content, SNCR denitration can meet the requirements of ultra-low emission, and the denitration efficiency of SNCR is above 80%. The author also found that the denitration efficiency decreases with the increase of oxygen content. The results show that the proposed “low NOx combustion +SNCR denitration” coupling technology scheme is feasible, the denitration efficiency of this scheme can reach more than 80 %, which can meet the requirements of low NOx emission in flue gas less than 50 mg/Nm3.
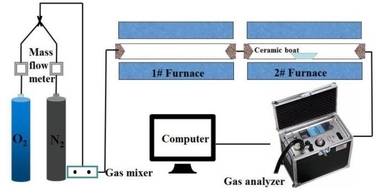
Hu [4] et al. used computational fluid dynamics to simulate the combustion and SNCR process of municipal solid waste in a mobile incinerator by analyzing combustion characteristics with temperature, NOx emission, NOx removal efficiency and NH3 escape. The author finally determined the optimal SNCR injection location, injection speed and injection ratio. It is found that the injection velocity and injection ratio of SNCR at the nozzle position have almost no influence on the furnace temperature and O2 concentration. Under the conditions of different injection velocities and ratios of SNCR, the temperature of the SNCR injection zone in the range of 800 °C ~ 1100 °C, the result of research shows that the injection location has a great influence on the removal efficiency of NOx and NH3 escape. The best ammonia injection location is in the layer and in the front region, the best injection speed is 13.80 m/s, under which condition the NOx removal rate is 51.50 % and NH3 escape is 11.08 mg/Nm3.
In recent years, with the development of technology and the increasingly strict emission standards, the denitration efficiency of traditional SNCR technology is low, and it is difficult to meet the requirements of ultra-low emissions. Moreover, SNCR technology has the risk of secondary pollution caused by ammonia escape due to high temperature.
4 SCR
Selective catalytic reduction (SCR) denitration technology is researched and developed by the United States Engelhard company, which means the process that NOx reduces into harmless N2 by the reducing agent (urea, NH3, H2, CO, etc.). NH3-SCR is the most widely used and mature technology currently, and is also recognized as the worldwide denitration technology with the best effect.
V2O5-WO3(MoO3)/TiO2 catalyst is the most commonly used catalyst for NH3-SCR. Among V2O5-WO3 (MoO3)/TiO2 catalysts, V2O5 is the active species of reaction, anatase TiO2 is the catalyst carrier, and WO3(MoO3) is the reaction promoter, which is used to stabilize the catalyst structure and inhibit the catalyst sintering. However, due to the relatively high denitration reaction temperature for V2O5 catalyst, some manganese-based catalysts have also been reported for low-temperature NH3-SCR technology. In recent years, the research of NH3-SCR technology focuses on reducing the reaction temperature and improving the sulfur resistance.
Murayama [5] et al. synthesised unloaded VOx for denitration reaction. The results show that the catalyst can achieve high-efficiency conversion of NO below 150 °C. The author used vanadium oxalate (IV) as the vanadium salt precursor, and roasted it at low temperature. VOx with different defect concentrations was constructed by controlling the roasting time. Among them, the sample calcined for 2 h shows the best SCR activity. Under the reaction space velocity of 40 000 ml/(h·g), the NO conversion rates at 100 °C and 150 °C were 80 % and 100 %, respectively.
Lian [6] et al. prepared Nb-doped VOx/CeO2 catalyst for denitration reaction. During the sulfation process, the Nb-VOx/CeO2 catalyst adsorbs more nitrate than sulfate, so the catalyst still maintains high activity of denitration. If O2 is introduced at the same time as SO2, active nitrate is no longer formed, and the catalyst activity is also significantly reduced. The main contribution of Nb to the sulfur resistance of the catalyst is to promote the L-H reaction.
Chen [7] et al. synthesized Mn1Fe0.25Al0.75Ox catalyst by calcining the LDH precursor of Mn1Fe0.25Al0.75 -NO3. This catalyst can exhibit high denitration efficiency from 75 °C to 300 °C. At the space velocity of 60 000 h-1, NOx conversion rate exceeds 90 %. The results show that the addition of Fe can significantly improve the sulfur resistance of the MnAlOx catalyst. In the presence of 100 ppm SO2, the denitration efficiency of the Mn1Fe0.25Al0.75Ox catalyst can still be as high as 76.6 % after 12 h stability test. The author speculates the reason may be that the addition of Fe increases the surface acidity, the mobility of surface oxygen and the amount of surface adsorbed oxygen (Oβ), which promotes the adsorption of NH3 and the reduction of NO. In addition, Fe can reduce the formation rate of sulfate species. In-situ FT-IR analysis confirmed that the Mn1Fe0.25Al0.75Ox catalyst has less sulfate formed on the surface and is less affected by SO2.
The CO in the exhaust gas that not fully burned can be used as a reducing agent in Co-SCR technology. In the absence of any additional reactant supply, the CO-SCR method removes two kinds of harmful gas (NO and CO) in the industrial waste gas at the same time. Using CO as reducing agent can not only reduce the denitration cost, but also avoid other harm to the system. The main reaction equation of CO-SCR is: 2NO+2CO=N2 +2CO2. Noble metals such as Pt, Pd, Rh and Ir are often used as active components of catalysts. But due to their high price, the research focus has gradually shifted to transition metal catalysts in recent years.
Wang [8] et al. synthesized active semi-coke (ASC) supported Fe and Co catalysts by the hydrothermal method and used them in CO-SCR. The authors studied the activity of catalytic materials and their sulfur/water resistance. It is found through experiments that the Fe0.8Co0.2/ASC catalyst has the best denitration performance. The author believes that the formation of Brønsted acid sites and oxygen vacancies improves the redox performance. The author also studied the effect of water and SO2 on the catalytic activity and found that when only water is present, the denitration performance is reduced due to the competitive adsorption of water and NO. When only SO2 is present, the denitration efficiency is reversibly reduced; however, when water and SO2 are both present, the catalyst will be irreversibly deactivated.
T. Boningari [9] et al. synthesized Mn/TiO2 catalyst and used it for CO-SCR reaction. The results show that the effect of Lewis acid site on the catalyst surface was greater than that of Braunst acid site. The adsorbed NO needed to be activated and dissociated at Mn ion site, and then reduced to intermediate N2O, which then reacted with CO to produce the final product N2. In the reaction process, the presence of NO inhibits the oxidation of CO to CO2, and excessive O2 does not inhibit the reduction of NO, so the denitration efficiency is still high under the condition of rich oxygen.
Yang [10] et al. synthesized octahedral Cu-BTC and Ce-Cu-BTC catalysts by solvothermal method for Co-SCR. Studies show that the addition of Ce-Cu-BTC contributes to improving the catalytic performance of the Co-SCR reaction and NOx adsorption capacity, because there are more oxygen vacancies on the surface of Ce-Cu-BTC. The author also finds that in typical CO-SCR, absorbed NO species are converted into N2 by reacting with absorbed CO, and the reaction of CO2 follows the Langmuir Hinshelwood (L-H) reaction mechanism.
However, when NH3 is used as a reducing agent, due to its strong corrosiveness, it has high requirements on pipelines and equipment. The use of H2 as a reducing agent does not produce any secondary pollution, which has attracted the attention of various research institutions. H2-SCR mostly uses precious metal catalysts, and the starting temperature of reaction is generally lower (generally less than 150 °C), so energy consumption will be greatly reduced.
Zhang [11] et al. synthesized binary layered double hydroxide (LDH)-derived mixed metal oxide supported Pt as an H2-SCR catalyst for denitration performance research. The Pt/M3Al1Ox (M=Co, Mg, Ni or Cu) catalysts were characterized and systematically evaluated in detail under the conditions of 120~300°C and presence of oxygen. All catalysts exhibited high H2-SCR activity, and the denitration efficiency is higher than 90% at 200-220 °C. Among them, the 0.1Pt/Mg3Al1Ox catalyst has the highest NOx conversion rate, and the 0.1Pt/Ni3Al1Ox catalyst has the highest N2 selectivity. The author believes that this work can provide a new H2-SCR catalytic material choice.
The denitration technology using hydrocarbons as reducing agents, as an alternative technology to the NH3-SCR method, can also avoid secondary pollution caused by ammonia leakage and ammonia escape, and can achieve simultaneous removal of NOx and hydrocarbons in flue gas. It has received extensive attention from many scholars and has development potential in the world. Many CH compounds such as alkanes, alkenes or alcohols can be used in the HC-SCR reaction, but the main problem of this process is that the reaction temperature is generally high. Also, if the tail gas does not originally contain reducing hydrocarbon compounds, it will increase the emission of carbon dioxide, which causes secondary pollution.
Xu [12] et al. synthesized Ag/Al2O3 catalyst for ethanol-SCR. The results show that in the absence of water vapor, the NOx removal efficiency is negatively correlated with temperature. The author found that ethanol was partially oxidized at a low temperature below 400 °C to produce enols and acetate through in-situ characterization and the former has much higher NOx reduction activity than the latter. At temperatures above 400 °C, only acetate appears during the partial oxidation of ethanol, and its further reaction with NOx is a high-temperature route. More importantly, the introduction of water vapor significantly improves the denitration activity of Ag/Al2O3 on ethanol-SCR, especially in the low temperature region.
Lu [13] et al. proposed a new type of sulfur cycle integrated H2S (H2S-SCDD) synchronous catalytic technology for desulfurization and denitration. The previously reported H2S-SCDD process requires a higher operating temperature (over 600 °C). In order to realize the low-temperature operation of the H2S-SCDD process, the author prepared Al2O3-TiO2 catalysts supporting different transition metal oxides to be tested. The results show that the CeO2-AT catalyst is a suitable catalyst for the H2S-SCDD process, which can react at 300~400 °C. The increase of Ce loading can promote the elimination of NO but slightly inhibit the elimination of SO2. On 15% Ce-AT catalyst, the optimal temperature for the H2S-SCDD process is 240-280 °C. At 280 °C, the conversion rates of SO2 and NO are about 75% and 90%, respectively. Based on this, the author believes that the sulfur recycling process is a promising and attractive industrial flue gas treatment technology.
The advantage of SCR technology is high denitration rate, but the disadvantage is that the SCR process is easy to produce N2O gas. And also most of the catalysts are transition metal oxides or precious metals, so the cost is high. In addition, sulfur or dust in the flue gas can easily lead to catalyst poisoning or blockage.
5 Photocatalytic denitration
Photocatalytic denitration technology is divided into photocatalytic oxidation denitration technology and photocatalytic reduction denitration technology. Titanium dioxide, Bi-based oxyhalide materials and metal-organic frameworks have been widely studied as photooxidation catalysts for their good stability, wide sources, low price and high photooxidation efficiency.
Lu [14] et al. synthesized Bi/Bi2O2-xCO3 as photocatalytic oxidation catalyst through the plasma method and carried out a test to remove NO. The experimental results show that under visible light irradiation, the best NOx removal efficiency of the catalyst was as high as 50.5%, which exceeded that of commercial photocatalysts. At the same time, it can inhibit the formation of harmful by-product NO2 (selectivity is 98%). A toxicity assessment was also conducted, which proved the material‘s good biocompatibility. The author believes that this study provides an effective surface engineering strategy for the preparation of highly reactive and selective photocatalysts.
Wang [15] et al. synthesized the size-matched Sb2WO6/BiOBr photocatalyst by the precipitation-deposition method at room temperature and used it for photocatalytic oxidation denitration. Under visible light irradiation, the prepared Sb2WO6/BiOBr photocatalyst has significantly higher NO removal efficiency than pure Sb2WO6 and BiOBr. The research results show that Sb2WO6 has the best photocatalytic performance when the proportion of Sb2WO6/BiOBr composite is 30%. Even if it is recycled 5 times, it still maintains good denitration performance. Not only that, the author also used in-situ drift to analyze the evolution of reaction intermediates over time in the process of photocatalytic oxidation of NO. The author believes that the improvement of the denitration performance of the Sb2WO6/BiOBr composite photocatalyst may be related to the interaction between BiOBr and Sb2WO6 and the s-type charge transfer path.
Metal-organic framework materials (MOFs) have received widespread attention in the field of photocatalysis due to their high specific surface area. The disadvantages of MOFs are invisible light response and low carrier migration efficiency. Wei [16] et al. combined CQDs and MOFs for visible light photocatalysis of NO. The author synthesized CQDs/ZIF-8 composite by a simple dipping method. The 0.5-CQDs/ZIF-8 composite has the best NO removal performance, which is about 43%. The research results show that compounding with CQDs is an effective way to improve the visible light catalytic activity of ZIF-8, and CQDs improves the visible light utilization rate and carrier separation efficiency. The author also proposed a possible mechanism for removing NOx from CQDs/ZIF-8 composites.
Photocatalytic reduction refers to the reduction of NOx to harmless N2 under the action of light and catalyst. In the reduction process, additional reducing agents such as ammonia or alcohols need to be added. Comparing with photocatalytic oxidation, photocatalytic reduction does not generate nitrate, and does not require frequent washing and regeneration.
In recent years, the photocatalytic reduction technology at lower temperature has aroused widespread interest in academia. Li [17] et al. synthesized Pr1-xCexFeO3/palygorskite (Pal) nanocomposites by the in-situ sol-gel method and used them for photocatalytic reduction. The test results show that when x<0.05, Pr1-xCexFeO3, as a perovskite solid solution, can be uniformly loaded on the surface of the pal. The author studied the effect of Ce doping on NO conversion rate and N2 selectivity under visible light irradiation, and found that Pr0.7Ce0.3FeO3/Pal catalytic material has the best effect, for which the NO conversion rate is 92% and N2 selectivity is 99%. The author also studied the sulfur and water resistance of the catalyst. The results show that the addition of Ce is beneficial to the formation of cerium sulfate and can inhibite the sulfation of NH3 by SO2. Pal can inhibit the corrosion of the catalyst by H2O.
Nguyen [18] et al. synthesized molybdenum-doped titanium dioxide nanotubes and studied their special photocatalytic reduction activity for NO2 and CO2 gases. Among these catalytsts, titanium dioxide nanotubes are synthesized by the hydrothermal method, and Mo is doped by three methods: hydrothermal method, precipitation method and immersion method. The experimental results show that the doping of Mo has no significant effect on the morphology, microscopic results and crystal structure, but it changes the electronic band structure of the material and the acidity/basicity of the surface to a certain extent. The author also found that the doping of Mo reduces the oxidation ability of the material but enhances its reducing ability. The Mo-doped catalytic material prepared by precipitation method has the strongest reducing ability, which may be due to the valence of Mo which is Mo4+ and Mo5+.
It is worth noting that there have been a few reports on the practical application of photocatalytic denitration, but the industrial application of this technology still faces many research challenges. For example, photocatalytic oxidation converts NO into HNO3 through the formation of HNO2 and NO2, which covers the surface of titanium dioxide and causes the catalyst to deactivate. So the catalyst requires frequent washing and regeneration. The main problem of photocatalytic reduction technology is that other reduction by-products may be produced during the reaction. The technology is currently still at the laboratory research and development stage. Therefore, in order to achieve industrial applications, in-depth research is still required in the future to broaden the visible light absorption spectrum of the catalyst, improve the photo-oxidation efficiency, reduce the cost of the catalyst and increase the service life of the catalyst.
6 Oxidation absorption method
The oxidation absorption method means that insoluble NO was oxidizes into high-valence nitrogen oxides (such as NO2) that are easily soluble firstly in the absorption liquid, and then promotes the denitration reaction. Plasma technology (pulse corona, electron beam, dielectric barrier discharge), gaseous oxidant (O2, Cl2, ozone, ClO2) or oxidant solution (Fenton reagent, chlorine-containing oxidant, persulfate, KMnO4, KBrO3, K2CrO7, Na2CrO4), etc. can be used to oxidize NO, and for subsequent operations. The more commonly used oxidants are ozone and hydrogen peroxide, because the oxidation products of them are oxygen and water respectively, which will not cause additional pollution to the absorption liquid. Oxidation absorption technology is often used for simultaneous desulfurization and denitration.
Liang [19] et al. combined dielectric barrier discharge (DBD) and rotating packed bed (RPB) for flue gas denitration reaction for the first time. This technology uses DBD to oxidize part of NO to soluble NO2 first, and uses (NH2)2CO as the absorbent. NaClO2 is an effective additive, which is beneficial to the subsequent absorption process of RPB. When the NOx concentration is 1000 ppm, the NOx conversion rate can reach more than 50%. The experimental results show that the removal efficiency (η) of nitrogen oxides increases greatly with the increase of the oxidation degree of nitrogen oxides. In addition, with the increase of NaClO2 concentration and RPB rotation speed, the NO conversion rate also increases. Under the best process conditions, the denitration efficiency can reach more than 85%. The author believes that the combination of DBD and RPB flue gas denitration technology has broad application prospects.
As a gaseous oxidant, ozone is widely used in oxidation absorption denitration technology. Zou [20] et al. studied the synergistic technology of ozone and magnesium oxide, and used it to simultaneously eliminate nitrogen oxides and sulfur dioxide. The influence of O3/NO molar ratio, oxidation temperature, oxidation residence time, etc. on N2O5 decomposition and O3 consumption distribution is systematically analyzed. This work is of great significance for improving denitration efficiency and reducing O3 cost. The test results show that when the O3/NO molar ratio is greater than 1, the yield of N2O5 is the highest at 90 °C. When the molar ratio is 1.8, the denitration efficiency reaches 96.5%. The author also found that the denitration efficiency decreases with the increase of SO2 concentration, and the addition of MgSO3 to MgO slurry can promote the absorption of NO2. Yang [21] et al. studied a new type of liquid catalyst (tributyl phosphate, TBP) for NO ozone oxidation process, and finally simultaneous removal of NOx and SO2. The author studied the absorption process and reaction mechanism of NO and SO2 in a small-scale test system, and studied the removal efficiency and key parameters in a pilot-scale test. The test results show that after adding TBP, the removal efficiency of NO2 rises from 20~40% to more than 90%. The author believes that this addition strategy has great potential in industrial applications for simultaneous removal of NO and SOx.
Han [22] et al. studied the influence of ethanol injection for NOx advanced ozone oxidation based on a scale-up reaction system. The author found that adding ethanol to the simulated flue gas can effectively improve the denitration effect. At 150 °C, the initial NO concentration is 400 ppm, and 200 ppm O3 can oxidize about half of the NO. When 150 ppm ethanol vapor was introduced again, the outlet NO concentration was reduced to 68 ppm. The author believes that this phenomenon was due to the synergistic reaction between ozone, ethanol and NOx. After analysis, it is found that ethanol and O3 produce a large number of organic free radicals and hydroxyl free radicals in the presence of NOx.
Xu [23] et al. studied the use of ClO2 gas phase oxidation combined with CaCO3 slurry absorption process in the spray tower to improve the desulfurization and denitration efficiency, and also reduce the treatment cost. The author conducted an experimental study on the optimal test conditions and system stability of the combined process. The optimal reaction conditions of ClO2 gas phase oxidation combined with CaCO3 slurry absorption process are determined through experiments: ClO2/NO=0.7 (molar ratio), the initial pH of the CaCO3 slurry is 7.0, the temperature is 55 °C and the liquid-to-gas ratio is 18 L/m3. When initial NOx concentration is 509 mg/m3, the removal rate reaches 81%. The technology can simultaneously desulfurize, the total operating cost is low, the floor space is small and it has a good promotion prospect.
However, gas-phase oxidants are expensive and relatively difficult to transport, and there is a risk of leakage. Therefore, the method of adding an oxidant in the liquid phase has been used recently to gradually replace the gas-phase oxidant.
Fenton reagent is the most commonly used nitrogen oxide liquid oxidant. Liu [24] et al. used Fe2(MoO4)3 as the hydrogen peroxide decomposition catalyst to simultaneously desulfurize and denitrate in the flue gas. The author proposed a removal process in which pollutants are first oxidized by ·OH radicals and then absorbed by ammonia solution. In the article, the effects of hydrogen peroxide dosage, temperature, space velocity and flue gas composition are comprehensively studied. The experimental results show that under the optimal reaction conditions, the removal efficiency of SO2 is 100%, and the removal efficiency of NOx is 91.4%. The author also found that the presence of SO2 is more conducive to the removal of NOx, because SO2 can promote the oxidation of NO. The characterization results show that NH4NO3 and (NH4)2SO4 are the main reaction products. The author also initially established a three-step reaction mechanism for the simultaneous removal of the H2O2/Fe2(MoO4)3 system.
Song [25] et al. proposed Fe2.5M0.5O4 (M = Mn, Ti and Cu) as a hydrogen peroxide decomposition catalyst to simultaneously remove SO2 and NO through catalytic oxidation absorption for the first time. The research results show that the removal efficiency of NO is affected by the catalyst, hydrogen peroxide consumption, reaction temperature, space velocity and other coexisting gases. The author also found that the doping of Mn, Ti and Cu in magnetite is beneficial to the removal of NO. The reason may be that the doping of these three elements can increase the surface area of the catalyst; Ti on the surface can promote the decomposition of hydrogen peroxide; the doping of Mn and Cu can promote the generation of oxygen vacancies and thus increase the activity. The author also proposed two layouts for industrial applications based on the process.
In order to reduce the cost of hydrogen peroxide catalyst, fly ash can be used as a raw material for modification to prepare the catalyst. Yang [26] et al. used power plant fly ash as raw material to prepare high-efficiency and low-cost hydrogen peroxide catalysts for desulfurization and denitration through grinding, alkali modification, and magnetic separation. The experimental results show that the removal effect is the best when the molar ratio of hydrogen peroxide to NOx is 3, and the removal efficiency of NOx can reach 80%. The author also found that the iron content in fly ash is the main factor affecting the denitration efficiency. The magnetic separation can increase the iron content, and the addition of alkali is beneficial to the magnetic separation process. In another article by the author [27], the catalyst was also prepared by alkali-magnetic modification with fly ash as the raw material. First, the fly ash raw material is modified by calcium hydroxide to destroy the vitreous shell to dissolve the internal oxides, and then magnetic separation and roasting to obtain a highly reactive catalyst. The author carried out desulfurization and denitration in a fixed bed reactor, and used an ultrasonic atomizer to atomize hydrogen peroxide. Under the conditions of hydrogen peroxide concentration 1 mol/l and flow rate 0.03 ml/min, 100% desulfurization efficiency and 90% denitration efficiency can be achieved.
Using chlorite as an oxidant to simultaneously remove SO2 and NO is an effective desulfurization and denitration technology, but its large amount leads to higher costs. Hao [28] et al. used the triple zone control method to explore whether it is possible to reduce the amount of chlorite and reduce costs while ensuring the efficiency of NO removal. The reaction system includes a NaClO2 solution, an aqueous layer for capturing ClO2 released from the NaClO2 solution, and a Na2SO3 solution. The research results show that the co-existence of SO2 and O2 has a synergistic effect on the removal of NO, but the presence of HCO3- inhibits the removal of NO.
Han [29] et al. used the technology of circulating washing and online supplement of NaClO2 solution to simultaneously desulfurize and denitrate in the simulated flue gas. The author studied the influence of operating parameters on the removal effect in detail, and found that the SO32- intermediate product produced by hydrolysis of SO2 can increase the absorption of NO2 to a certain extent. The author also found that adding a certain amount of ethanol to the washing liquid can effectively improve the removal performance of NOx, which may be due to the fact that ethanol has an inhibitory effect on the oxidation of SO32-.
The oxidation absorption method belongs to the category of wet denitration technology. It is simple and easy to implement, has a small area of equipment, and has a high denitration rate. However, it also has disadvantages such as complex products, difficult to re-use, and easy to cause secondary pollution. The products generally exist and are difficult to recycle.
7 Complex absorption method
The complex absorption method is to add a complex absorbent to the scrubbing liquid to react with NO, and generate a complex of NO, which converts NO in the gas phase into NO in the liquid phase, thereby improving the gas-liquid quality efficiency transfer of NO. Then the complexing agent can be regenerated and reused through electrolysis or other means. The center of commonly used complexing absorbents is often iron ions [30]. At present, the complexing agent is easily oxidized and it has not been industrialized.
Zhang [31] et al. studied Fe(II) EDTA complexation-NaSO3 reduction and absorption of NO. The authors found that the initial PH and Fe(II) EDTA concentration have a greater influence on the complex absorption of NO. It is not conducive when the pH is acidic or alkaline for the complexation and absorption of NO. Under low temperature and anaerobic conditions, it is more conducive to the complex absorption of NO, and the presence of SO32- significantly increases the absorption time of complex NO. Under the optimal conditions, the complex absorption of NO is 1.099 mol/mol, and the maximum removal efficiency of NO can reach 96.98%, which can be maintained above 90% in 570 min.
Zhu [32] et al. used Na2-EDTA and sodium citrate as ligands for the denitration reaction. The author found that the addition of sodium citrate not only improved the denitration reaction, but also reduced the economic cost. The experimental results show that the denitration efficiency is also related to temperature, PH value, and liquid-gas ratio. Under the best complex absorption conditions (mixed complex concentration is 0.05 mol/l, the temperature is 45 °C, and the liquid-to-gas ratio is 10, PH=6), the denitration efficiency can be as high as 85%.
Jun [33] et al. studied the use of FeII (EDTA) to capture NO and the use of a rotating drum biofilter to increase the vortex effect of the system. The author proposed the FeII (EDTA) denitration reaction in a biological drum filter and studied the NO mass transfer model. The long-term operation results show that the denitration rate of the reactor can be stabilized at about 95%, and the average relative deviation between the model and the experimental data is only within 2.27%. the author believes that all these findings can guide the design and operation in the industrial application.
The complex absorption method does not require the ratio of NO/NO2 in the flue gas, and the complex absorption liquid can be recycled, which has a good application prospect. The loss and regeneration of the absorbent are the main problems faced by the complex absorption method. The denitration process technology of the complex absorption method is not yet mature, and there is no industrial application case for the time being.
8 Biological denitrification
Biological denitrification refers to the use of microorganisms to degrade nitrogen oxides into simple and harmless inorganic substances or microbial cytoplasm in flue gas. The advantages are large scope of application, simple process equipment, low energy consumption and cost, high efficiency, and no secondary pollution. The biological method mainly includes two processes, namely mass transfer process and reaction process. Firstly, nitrogen oxides are transferred from the gas phase to the medium containing microorganisms, and then the NOx is removed on the medium through the metabolism of microorganisms in life activities.
Ma [34] et al. successfully removed both NO and CO2 in simulated flue gas by using the naturally isolated strain Ceratocystis PF3. PF3 has good environmental adaptability (pH: 4.5~10.5; temperature 15~30 °C) and good resistance to CO2 (10%-15%), NO (100-500 ppm), SO2 (50 ppm), and HSO3-(2 mM). The NO and CO2 removed from the biomass were 96.9±0.03% and 87.7±6.22%, respectively. These results indicate that the selected new microalgae strain-Ceratocystis PF3 is expected to achieve a win-win situation for flue gas treatment and flue gas carbon and nitrogen recovery.
Biological denitrification technology is a new technology with industrial application prospects. However, in the application process, due to the large proportion of NO in NOx in the flue gas, and NO is difficult to dissolve in water, the NO gas-liquid mass transfer efficiency is low. At the same time, the adsorption capacity of microorganisms for NO is not strong, and the environmental conditions in which the microorganisms live are difficult to control. The actual NOx purification effect is not ideal, and the technology is still at the research stage. In order to realize the industrialization of this technology as soon as possible, further research should be carried out in the following two aspects: improving the NO gas-liquid mass transfer efficiency and optimizing the conditions that are conducive to the microbial biochemical reaction process.
9 Coupled denitration
The use of a single technology for denitration has the problems of low denitration efficiency and inability to meet the emission requirements, and all of them have certain shortcomings. Therefore, the two technologies can be coupled to improve the denitration efficiency through synergistic technology.
Ultraviolet, microwave and electrochemical measures can also be used to assist oxidation absorption to improve nitrification efficiency. Hao [35] et al. developed a microwave-induced ultraviolet irradiation H2O/O2 technology for synergistic removal of NO and Hg0, with removal efficiencies of 89.3% and 99.5%, respectively. This technology can also desulfurize at the same time, the efficiency can reach 97%. The author found that O2 content in the range of 2%~8% can effectively remove NO and Hg0. Ozone and hydroxyl radicals are the main oxidants in the reaction. The test results show that high temperature can promote the removal of NO, but inhibit the removal of Hg0. The presence of Cl-1 and Br-1 inhibited the removal of NO and promoted the removal of Hg0. Wang[36] et al. developed a microwave-activated H2O2/persulfate (PS) dual oxidant system for denitration reaction, and used this system to verify and characterize several basic issues of NO removal. The author found that SO4-•, •OH, HO2•, H2O2 and PS are the active substances for removing NO, of which •OH occupies the main role. The test results also show that increasing the microwave power, PS and H2O2 concentration, and O2 content can enhance the NO removal efficiency, while increasing the NO and SO2 concentration, solution pH and flue gas flow rate will reduce the NO removal efficiency. Liu [37] et al. developed a semi-dry microwave activated persulfate system for simultaneous desulfurization and denitration for the first time, and analyzed the feasibility of this technology by analyzing the reaction mechanism, testing and characterization. The author found that the increase of microwave radiation power, persulfate concentration and O2 concentration can enhance the removal efficiency of SO2 and NO, while the increase of NO and SO2 concentration has the opposite effect. At the end of the article, the author also proposed the process application of this technology and the post-processing route of the product.
Hao [38] et al. studied the process of oxidizing NO with chlorite (NaClO2) catalyzed by ultraviolet light (UV). Using this process, the conversion efficiency of NO is 98.1%. The research team also proposed a process to simultaneously remove SO2 and NO by using this system [39]. The production of ClO2 and NO2 was suppressed by adding ammonia water. Using this process, the removal rates of SO2 and NO reached 98.7% and 99.1%, respectively. The author found that stronger ultraviolet light intensity and shorter wavelength can enhance the removal of NO, but it is not sensitive to the changes of pH and temperature. The author believes that the mechanism of removing SO2 and NO is considered to be the synergistic effect of acid-base neutralization and free radical oxidation. Another paper [40] by the same author proposes a method to synergistically remove SO2 and NO by UV-heat-H2O2, and then recover the nitrogen and sulfur resources in the flue gas. First, the initial absorbent ammonia water is used to pre-absorb SO2; then, a UV-heat-H2O2 hybrid catalytic reactor is used to oxidize NO with hydroxyl radicals; finally, the produced NO2 and other products are absorbed in the main absorber. Using this method, the removal rates of SO2 and NO reached 99.3% and 96.3%, respectively. Electron spin resonance (ESR) results show that compared with pure thermal catalysis, UV-thermal hybrid catalysis produces HO• more effectively.
Through a pilot-scale photochemical spray tower, Xie [41] et al. studied the technology of using vacuum ultraviolet (VUV) light activated H2O2/urea to simultaneously remove SO2 and NO in flue gas. The author also studied the influence of different operating variables on the removal efficiency and analyzed the reaction mechanism, and finally looked forward to the prospects of the new technology. The experimental results show that with the increase of vacuum ultraviolet radiation intensity and hydrogen peroxide concentration, the denitration efficiency gradually increases. The removal concentration of NO first increases and then decreases with the increase of urea concentration and liquid-gas ratio. IC results show that NO3- is the removal product of NO. From VUV activation of H2O2, •OH plays a key role in removing NO.
Huang [42] et al. studied the technology of removing NO by electrochemical catalytic oxidation in the K2S2O8 system. The research results show that the efficiency of electrochemical catalytic oxidation denitration is higher than thermal activation, ultraviolet activation and transition metal activation K2S2O8, and the denitration efficiency of Ti-based IrO2 electrode can be as high as 89.3%. The author also found that at different pH values, the removal efficiency of NO always remained above 80%, proving that this removal technology has a wide range of universal applicability in industrial applications.
Gong [43] et al. first proposed a MOFs-NTP technology for denitration research. The author synthesized the MOFs material CuBTC through the microblog method and combined with the non-thermal plasma for synergistic denitration. The removal efficiency of NO can be as high as 97.87%, which is an increase of 76.77% and 64.43% compared with the use of MOFs and NTP technology, respectively. Through characterization studies, it is found that CuBTC can be activated by NTP and generate Cu(I)/Cu(II) sites with coordination unsaturated, which greatly improves the surface catalytic activity of CuBTC. The author believes that MOFs as ultra-efficient gas pollution adsorption materials will have broad applications in the field of environmental protection. In addition, Wang [44] et al. used a non-thermal plasma coupled γ-Al2O3 adsorbent mixing system for collaborative desulfurization and denitration. The research results show that the presence of NTP can significantly improve the NOx adsorption effect of γ-Al2O3.
In order to improve the efficiency of denitration, Zhang [45] et al. integrated chemical absorption and biological reduction technology for denitration. The author developed a two-stage CABR system, which has better antioxidant capacity and higher NO removal load. The research results show that when the FeEDTA concentration is higher than 4 mmol/l, the NO removal efficiency can maintain >90%.
In order to improve the removal efficiency of nitrogen oxides, the combination of absorption technology with rotating packed bed and microbubble technology which was used to improve the gas-liquid mass transfer efficiency and contact area were reported in recent years. Cai [46] et al. proposed a new technology to remove nitrogen oxides through oxidation absorption in a rotating packed bed (RPB) reactor. The author found that RPB has high gas-liquid mass transfer efficiency. By using a stirred tank reactor (STR) and an RPB reactor in series, more NO can be oxidized to NO2, which promotes the dissolution of nitrogen oxides and improves the subsequent absorption effect. Compared with the traditional urea/sodium chlorite composite absorbent in the RPB system, the denitration efficiency is increased by about 30%. Xiao [47] et al. proposed a micro-nano bubble gas-liquid dispersion system based on Na2SO3 reductant wet-cycle redox process for simultaneously removing NO and SO2 for the first time. Compared with traditional macro bubbles, ultra-micro bubbles can generate •OH with strong oxidizing ability, which improves the solubility of gas. As a reducing agent, Na2SO3 can reduce higher valence nitrogen oxides to non-toxic and harmless nitrogen. At the same time, the effects of operating time, initial pH and temperature of the absorbent and the concentration of Na2SO3 on the removal of NO and SO2 were studied. The author believes that this technology can achieve higher efficiency in the removal of NO and SO2, treat NO2- and NO3-, and reduce the cost of subsequent wastewater treatment. The main product sulfate can also be recycled, which is an economic and environmental protection exhaust gas purification technology. Si [48] et al. designed and tested a new technology based on ozone oxidation (spray-dispersed bubble technology), which can be used for simultaneous desulfurization and denitration in power plants. This technology combines the advantages of jet bubble reactors and spray towers. First, the initial removal is carried out in the spray zone, and then further removal in the bubble zone. The author found that compared with a single technology, the removal efficiency of SO2 and NOx of this technology is significantly improved, and the removal efficiency of NOx is 18% higher than that of the spray tower and 11% higher than that of the bubble reactor. In order to verify the effect of industrial application, the author conducted a 30 h stability test of real flue gas in a pilot-scale test. This technology provides synergistic advantages in emission reduction and energy saving.
10 Conclusion
With the tightening of environmental protection policies, requirements for the total and concentration of NOx emissions in cement, coking and other industries have become increasingly strict. There is an urgent need to develop more economical, reliable and efficient denitration technologies to achieve emission standards. This article categorizes and elaborates some of the denitration technologies reported in the literature. But there will still be many problems to be solved in practical applications, and more research and development efforts in academic and industrial circles are needed to overcome and solve them.
For industrial flue gas denitration, considering investment and operating costs, system stability, etc., only SNCR, SCR, and oxidation absorption technologies have large-scale applications in industry currently. However, with the implementation of ultra-low emissions, the denitration efficiency of SNCR will not be able to meet the emission standards. For SCR technology, the focus of research and development is to develop low temperature, high efficiency, high sulfur resistance and low cost SCR denitration materials. For photocatalytic technology, the future research directions are to broaden the visible light absorption spectrum of the catalyst, improve the photocatalytic efficiency, reduce the cost of the catalyst and increase the service life of the catalyst. The oxidation absorption technology is simple, suitable for low-temperature flue gas denitration, but the current operating cost is high and we hope it can be more economical in the future. Biological denitration technology has not yet been reported on industrial applications due to its slow reaction speed and low denitration efficiency. The future research focus of biological denitrification is to select better strains, improve denitrification efficiency, process equipment design and so on. Coupling technology for denitrification has a good application prospect, and the research and development of technology can be carried out in the future to promote the early maturity of this technology.
Industrial flue gas denitration technologies are booming and a hundred schools of thought contend. All of the technologies have their own advantages and applicable fields. We believe that through the joint efforts of industry and academia, in the near future, there will be more efficient, low investment and operating costs, and environmentally friendly denitration technology that enables large-scale industrial applications.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.


![1 NOx emissions of China from 2013 to 2019 [1]](https://www.zkg-online.info/imgs/1/8/3/3/1/1/1/tok_f20315c14740b96caf027ac5423e851c/w300_h200_x600_y366_Process_Lin_Zhao_Sinoma_Figure_1-0b6ceade6627a00b.jpeg)
![2 Fixed Industrial Resources NOx emissions of China from 2013 to 2019 [1]](https://www.zkg-online.info/imgs/1/8/3/3/1/1/1/tok_22ad73b64a7e8d711fc5024e7ce77326/w300_h200_x600_y360_Process_Lin_Zhao_Sinoma_Figure_2-7e6d7844639594f2.jpeg)
![3 NOx emission from exhaust gas of centralized pollution treatment facilities of China [1]](https://www.zkg-online.info/imgs/1/8/3/3/1/1/1/tok_a14fddacdfd887d9831332b4d6c98975/w300_h200_x600_y360_Process_Lin_Zhao_Sinoma_Figure_3-18e4dc981b3cb531.jpeg)
![5 NOx removal efficiency and NH3 slip [4]](https://www.zkg-online.info/imgs/1/8/3/3/1/1/1/tok_c029aa79cc35429762c302f14f48980d/w300_h200_x600_y339_Process_Lin_Zhao_Sinoma_Figure_5-49bc511a5fddd315.jpeg)
![6 a) The influence of SO2 concentration (0, 50, and 100 ppm) on the NOx conversion of Mn1Fe0.25Al0.75Ox, Mn1Al1Ox, and 8 wt% Mn-8wt% Fe/γ-Al2O3 catalysts at 150 °C in 1h; b) The long-term isothermal NOx conversion of Mn1Fe0.25Al0.75Ox, Mn1Al1Ox, and 8 wt% Mn-8wt% Fe/γ-Al2O3 catalysts at 150 °C in the presence of 100 ppm SO2. Reaction condition: 0.15 g catalyst, total flow rate =200 ml/min, [NH3]= [NOx]= 500 ppm, [O2]= 5%, [SO2]= 50 or 100 ppm (when used), Ar = balance, GHSV=60000 h-1 [7]](https://www.zkg-online.info/imgs/1/8/3/3/1/1/1/tok_43f98ab08c1fbdee97670dcb32979b79/w300_h200_x600_y245_Process_Lin_Zhao_Sinoma_Figure_6-b1273e0115180c55.jpeg)
![7 Schematic illustration of the synthesis process for the Ce-Cu-BTC catalyst [10]](https://www.zkg-online.info/imgs/1/8/3/3/1/1/1/tok_d8dac23024755f1b94ff4e8f364e4a64/w300_h200_x600_y214_Process_Lin_Zhao_Sinoma_Figure_7-1b5742e947495617.jpeg)
![8 Schematic illustration of charge transfer in the Bi/Bi2O2−xCO3 system and the possible mechanism of photocatalysis [14]](https://www.zkg-online.info/imgs/1/8/3/3/1/1/1/tok_08a120b04e07a372af69e47aae2ddc17/w300_h200_x504_y307_Process_Lin_Zhao_Sinoma_Figure_8-0f11fdbbd8453cf0.jpeg)
![9 Possible mechanism for photocatalytic reduction of NO2 and CO2 by Mo-doped TNTs [18]](https://www.zkg-online.info/imgs/1/8/3/3/1/1/1/tok_ee2a9fc936e25df8b3a1c735a2e62ac1/w300_h200_x501_y377_Process_Lin_Zhao_Sinoma_Figure_9-cfc2af2596decab5.jpeg)
![10 a) NO2 absorption performance under different systems; b) Effect of SO32- concentration on the NO2 removal performance; c) Effect of SO2 concentration on the NO2 removal performance; d) SO32- accumulation characteristics [21]](https://www.zkg-online.info/imgs/1/8/3/3/1/1/1/tok_e2849c57077e664113e6aa42d3f7f988/w300_h200_x600_y463_Process_Lin_Zhao_Sinoma_Figure_10-07ce63dce38b17ed.jpeg)
![11 The arrangement forms of simultaneous removal system: (A) Fixed bed reactor and (B) Cyclitic injection [25]](https://www.zkg-online.info/imgs/1/8/3/3/1/1/1/tok_5898cf66caf6ad505d7deef06008caa0/w300_h200_x501_y413_Process_Lin_Zhao_Sinoma_Figure_11-0b8c148c1bfe49e7.jpeg)
![12 Profile of NO removal efficiency and elimination capacity after start-up of RDB [33]](https://www.zkg-online.info/imgs/1/8/3/3/1/1/1/tok_6f6b34c60e58a960b1d15cf0676ff889/w300_h200_x405_y305_Process_Lin_Zhao_Sinoma_Figure_12-e781c5ba8825dffb.jpeg)