Research into the hydration kinetics of calcium aluminate cement and of calcium aluminate cement that contains spinel
Calcium aluminate cement (CAC) is well known for its rapid hardening, early strength, impermeability, resistance to corrosion and other advantages. Calcium aluminate cement that contains spinel is excellent, with high temperature performance, impermeability and durability. Calcium aluminate cement containing spinel (HAC) is the residue obtained by a special process after extraction of Ti-Si-Fe alloy from blastfurnace slag with a high titanium content. A kinetic model is established for the hydration of CAC and HAC. Based on the model and the measured emission of heat of hydration under isothermal condition, equations are derived to simulate the three basic processes of the hydration of the two materials. These are nucleation and crystal growth (NG), interactions at phase boundaries (I) and diffusion (D). The kinetic factors, e.g. a, n and K, are determined. These are the reaction rate, reaction exponent and reaction rate constant, respectively.
1 Introduction
Commercial production of calcium aluminate cement (CAC) from bauxite and limestone has been developed by Lafarge, France, by a fusion method since 1908 [1].
CAC is subdivided into pure calcium aluminate cement and alumina cement containing small amounts of SiO2 and Fe2O3.
CAC, with its rapid hardening, early strength, impermeability, resistance to corrosion, etc. due to the high Al2O3 content, is commonly used in the refractory industry [2, 3]. Because the spinel can absorb FeO, MnO, TiO2 and V2O5, this improves the slag viscosity and slag penetration. High alumina cement (HAC)...
1 Introduction
Commercial production of calcium aluminate cement (CAC) from bauxite and limestone has been developed by Lafarge, France, by a fusion method since 1908 [1].
CAC is subdivided into pure calcium aluminate cement and alumina cement containing small amounts of SiO2 and Fe2O3.
CAC, with its rapid hardening, early strength, impermeability, resistance to corrosion, etc. due to the high Al2O3 content, is commonly used in the refractory industry [2, 3]. Because the spinel can absorb FeO, MnO, TiO2 and V2O5, this improves the slag viscosity and slag penetration. High alumina cement (HAC) containing spinel has a unique refractory performance, high-temperature resistance and durability [4, 5]. The growing interest in the utilization of high alumina cement containing spinel as a refractory material has attracted the attention of researchers in recent years.
High alumina cement containing spinel is generally produced with burnt dolomite and alumina (see ternary phase diagram of CaO-Al2O3-MgO) [6-9]. A.H. De Aza et al. concluded that the hydration process and dehydration behaviour of cement containing 43 wt.% CA, 15 wt.% CA2 and 42 wt.% MgAl2O4 is comparable with that of commercial calcium aluminate cement [10]. J.M. Auvray indicated that spinel was present as micro crystals in the calcium aluminate cement, which improved the corrosion resistance to molten slag and metals. Calcium aluminate cement containing magnesium aluminate spinel is better than conventional cal-cium aluminate cement [11]. E. Dourdounis and other researchers prepared high alumina cement using ferronickel furnace slag, limestone and low-grade bauxite by a smelting reduction process. This has a satisfactory compressive strength when compared with commercial high alumina cement [12].
This paper studies calcium aluminate cement containing spinel (HAC), which is the residue obtained by a special process after extraction of Ti-Si-Fe alloy from blastfurnace slag with a high titanium content. The chemical composition and mineral composition of HAC is similar to CAC. The early compressive strength of HAC is lower than that of CAC although the compressive strength of HAC increases gradually with longer curing times [13-15].
A kinetic model for the hydration based on research can reflect the macroscopic and microscopic chemical reaction mechanisms based on dynamic internal factors and external influences on the reaction rate and the direction of the reaction [16]. Krstulovic et al. [17] described a kinetic model for the hydration of cementitious materials. There are three basic processes during the hydration: nucleation and crystal growth (NG), interactions at phase boundaries (I) and diffusion (D). Some researchers have focused on the dynamics of hydration of CAC [18-20] but the dynamics of hydration of HAC are still not clear.
The kinetic model was researched for the hydration of CAC and HAC. Factors such as reaction exponent, reaction rate constant and other dynamics parameters were determined on the basis of the kinetic model and simulation curve. In the comparison of CAC and HAC, the hydration processes and mechanisms were analyzed to assist in the comprehensive utilization of HAC.
2 Experimental procedure
2.1 Raw materials
The principal raw materials were calcium aluminate cement CAC and calcium aluminate cement containing spinel (HAC). The CAC came from Henan Zhengzhou jia of the Aluminate Cement Co., Ltd. HAC comprised residues extracted from HTBF slag. The chemical compositions of the CAC and HAC obtained by chemical analysis (Thermo Elemental IRIS Advantage Radial ICP-AES) are shown in Table 1.
It can be seen that CAC and HAC consist mainly of Al2O3 and CaO. The content of Al2O3 and MgO in HAC is greater than in CAC. The content of CaO in HAC is lower than in CAC (Table 1). The basicity coefficient Am and the aluminium/silicon coefficient A/S were obtained by chemical analysis. The Am of the CAC is 0.85 while the Am of the HAC is 0.89. The higher Am value means less CA and more CA2 in the material. The A/S of the CAC is 7.19 and A/S of the HAC is 18.36. The value of the A/S is closely related to the physical properties. The higher value of A/S means better mechanical performance.
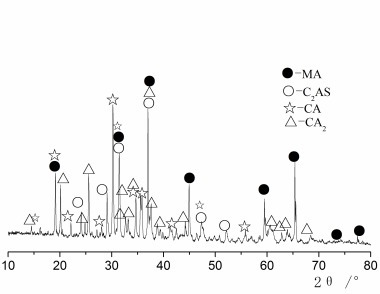
The mineralogical phases of the CAC and HAC were detected by an X-ray diffractometer (Philips, X’pert Pro) with Ni-filtered Cu Kα radiation (see Figure 1). The mineralogical phases are quite different. From Figure 1 it can be seen that the CAC contains a great deal of the CA and CA2 phases, and a little C12A7 can be detected, while for the HAC the main phases are CA and CA2, MgAl2O4 spinel and a little gehlenite (Figure 1).
2.2 Experiment methods
The HAC and CAC were ground to 4000 cm2/g in a 100 kg vibrating mill and measured by the Blaine method. The water consumption for standard consistency and the setting times were determined in accordance with the standard GB/T 201-2000. The paste compositions comprised standard sand, cement and water. Compressive strength tests were carried out on 40 mm x 40 mm x 40 mm test pieces at various ages.
The hydration behaviour of the CAC and HAC with a cement/water ratio = 0.35 was recorded by a microcalorimeter (eight channels, TAM Air C80, Setaram Company, France). The temperature was kept constant at 20.0 ± 0.5 °C during the measurements.
3 Results and discussion
3.1 Hydration properties of CAC and HAC
The results for standard consistency, setting time and the mechanical properties of CAC and HAC based on the standard tests are shown in Table 2.
From analysis of the results in Table 2 it can be seen that the water demand of the CAC to achieve standard consistency is greater than for the HAC. The initial setting time of the CAC is little shorter than that of the HAC, while the final setting time is longer. This means that the primary hydration speed of CAC is faster than that of HAC. Under standard curing conditions the compressive strength of CAC at 1 d is greater (44.3 MPa) than that of HAC (35.2 MPa). At 28 d, however, the compressive strength of HAC is higher than that of CAC. That means that the compressive strength of HAC clearly increases to a greater extent than that of CAC.
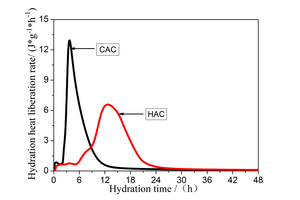
Cement hydration can consist of five stages: pre-induction and induction periods, acceleration period, deceleration period and stabilization period [21, 22]. From the curve showing the rate of heat release (Figure 2) it can be seen that the hydration of CAC takes place in several stages: (1) the induction period (0h ~ 2.13h), (2) the reaction accelerate period (2.13h ~ 3.72h), (3) the reduction reaction period (3.72h ~ 10.05h), (4) stabilization (> 10.05h). The exothermic hydration curve and the CAC differential hydration heat release curve are shown in Figure 2. This can also be divided into the following phases: (1) the pre-induction and induction period (0h ~ 5.25h), (2) acceleration period (5.25h ~ 12.78h), (3) deceleration period (12.78h ~ 21.55h), (4) stabilization period (> 21.55h). Analysis of the heat of hydration data shows that the maximum peak of the rate of evolution of heat of hydration of CAC appeared at 3.72h (12.93 J·g·h-1), and that of HAC appeared at 12.78 h (6.57 J·g·h-1). The induction period of HAC lasts longer than for CAC and the acceleration period means a longer reaction time, which is consistent with the results of the setting time.
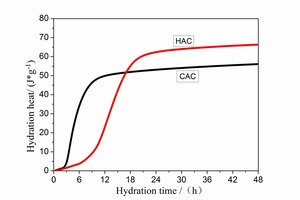
Figure 3 is the curve showing the amount of heat of hydration heat for CAC and HAC over 48 h. The cumulative hydration heat has been analyzed. The hydration heat of CAC and HAC before 2.35 h is low. Between 2.35h ~ 16.95h, the amount of hydration heat for CAC and HAC increases rapidly, in which the heat of CAC significantly greater than for HAC. The amount of heat for CAC and HAC is equal at 16.95 h (51.68 J·g-1). After 16.95h, the amount of heat from of HAC increases to more than that for CAC. At 48h the amount of heat from CAC is 56.06 J·g-1, and from HAC is 66.24 J·g-1.
According to heat of hydration data obtained by isothermal calorimetry it is possible to determine the degree of hydration relative to duration of hydration from equation (1). Where Qmax represents the total amount of heat a sample can release and Q(t) is the heat released by time t.
α(t)=Q(t)/Qmax ⇥(1)
The basic kinetic equations that describe the samples are based on the change in the degree of reaction relative to the time elapsed (α-t data), as shown by equations (2) and (3), where dα/dt is the degree of hydration, t0 is the time of the induction period, t50 is the time for the heat to reach 50 % of the maximum heat Qmax.
dα/dt=dQ/dt·⇥(2)
⇥(3)
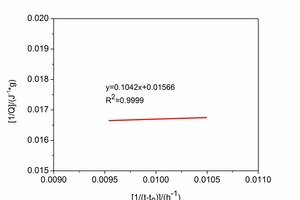
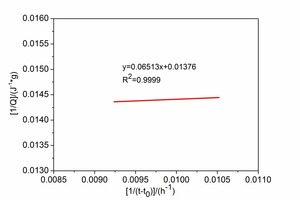
Figure 4 shows the maximum heat of hydration Qmax obtained by linear regression. Analysis of Figure 4 shows that Qmax for CAC and HAC is 63.86J·g-1 and 72.67J·g-1 respectively and the t50 values for CAC and HAC are 6.65 h and 4.73 h respectively.
According to the Krstulovic-Dabic model [23], the hydration reaction of CAC and HAC can be divided into three basic processes: nucleation and crystal growth (NG), interaction at phase boundaries (I) and diffusion (D) in accordance with equations (4)~(6). The values of n value usually range from 1 to 3; the exponent n describes geometrical crystal growth, where KNG, KI and KD are the rate constants for the reaction of nucleation and crystal growth, phase boundaries and diffusion, respectively. These three processes can all occur at the same time but the development of the hydration process depends on the slowest reaction process.
NG process: [-ln (1-a)]1⁄n = KNGt = F1(a)⇥(4)
I process: 1-(1-a)1⁄3 = KIt = F2(a)⇥(5)
D process: [1-(1-a)1⁄3]2 = KDt = F3(a)⇥(6)
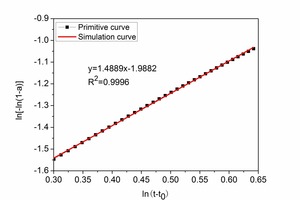
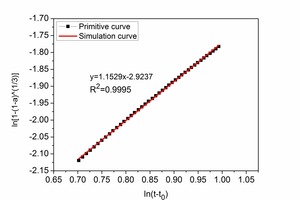
Figure 5 shows the diagram for determining the kinetic parameters. The NG, I and D processes are defined by the expressions (7)~(9) respectively.
NG process: ln[-ln(1-a)]=1.4889 ln(t-t0)-1.9882⇥(7)
I process: ln[1-(1-a)1⁄3]=1.1529 ln(t-t0)-2.9237⇥(8)
D process: ln[1-(1-a)1⁄3]2=1.7053 ln(t-t0)-5.2092⇥ (9)
The dynamic linear regression equation for HAC is the same as that for CAC. The regression equations are equations (10)~(12).
NG process: ln[-ln(1-a)]=1.4277 ln(t-t0)-1.9653⇥(10)
I process: ln[1-(1-a)1⁄3]=1.7578 ln(t-t0 )-4.4936⇥ (11)
D process: ln[1-(1-a)1⁄3]2=2.1409 ln(t-t0)-6.2891⇥(12)
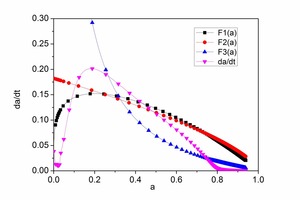
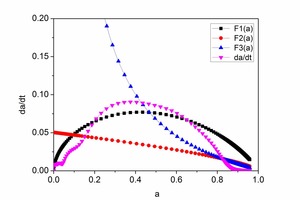
The hydration rate curves for CAC and HAC are shown in Figure 6. At early hydration times there are few hydration products of CAC and HAC and this is mainly controlled by nucleation and crystal growth (NG). As the hydration time increase there is an increase in the hydration products, ion migration is slow and the hydration reaction is controlled by the phase boundaries (I) or diffusion (D).
Analysis of Figure 6a and Figure 6b shows that the curves F1(α), F2(α) and F3(α) are well simulated by the dα/dt hydration rate curve, which is controlled by the nucleation and crystal growth (NG), phase boundaries (I) or diffusion (D), respectively. KNG, KI and KD are the rate constants with the values F1(α), F2(α) and F3(α). A larger rate constant means a faster hydration reaction rate. The point a1 is the intersection of F2(α) with F1(α), which means that the process changes from NG to I. The point a2 is the intersection of F2(α) with F3(α), which means that the process changes from I to D.
The kinetic parameters of CAC and HAC are given in Table 3. It is found that the value of KNG for CAC is less than that for HAC, which means that the hydration rate of HAC is faster during the NG process. The a1 of CAC is 0.2456, while the a1 of HAC is 0.09412. The initial setting time of HAC is longer than that of CAC. During the I and D processes, both the and KD of HAC are less than those of CAC. The a2, a2-a1 of CAC are 0.325, 0.7920 and the a2, a2-a1 of HAC are 0.0798, 0.6979. This means that for a long period the HAC is controlled by the phase boundary response. The ultimate cumulative heat of hydration of HAC is greater than that of CAC. For both CAC and HAC the values of KI and KD are significantly lower than KNG. KD was only 1/10 of KI, which means the nucleation rate was greater than the diffusion process and the hydration process is controlled by the diffusion during the attenuation period.
4 Conclusion
The maximum peak of the release rate of the heat of hydration for CAC occurred at 3.72 h (12.93 J·g·h-1), and that for HAC occurred at 12.78 h (6.57 J·g·h-1). The hydration reaction of CAC is sharp with a short duration, while that of HAC is slower and longer. The initial setting and final setting times of HAC are longer than those of the CAC and the cumulative quantity of heat from the HAC is greater.
According to the Krstulovic-Dabic model the hydration reaction of CAC and HAC can be divided into NG, I and D. During the early hydration period the CAC and HAC are both controlled by NG. During the late hydration the CAC is controlled by the NG and D processes for a long period and by the I process for a short period, while the HAC is mainly controlled by the I process.
Acknowledgements
This work was fully funded by the project effect of hydration mechanism and microstructure of Titanium tailings with dispersant-silicon power system (51802235) supported by National Natural Science Foundation of China and youth fund projects of the State Key Laboratory of Refractories and Metallurgy (Wuhan University of Science and Technology, 2016QN16).

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.