Reactivity and overburning tendency of quicklime burnt at high temperature
This study investigates the impact of different burning temperatures on quicklime slaking reactivity. Moreover, it reviews the state of the art of the effect of carbonate rock fabrics and calcination kinetics on the production of quicklime in industrial kilns. The burnability and overburning tendency of raw materials with different geological origin, stratigraphic positions, fabrics and compositions were investigated. Burning trials were performed on crushed rocks as received, between 1050 and 1300 °C, to simulate the soft-, medium- and dead-burning conditions occurring in Twin-Shaft Regenerative (TSR) kilns. The raw materials and burnt products were characterized based on a multidisciplinary analytical approach, including physical-chemical, mineralogical-petrographic, crystallographic and thermal analyses. The lime reactivity was determined according to the European slaking test method. By means of statistical analysis of different datasets, key factors controlling the lime reactivity and overburning tendency at high temperature could be identified.
1 Introduction
The reactivity of commercial lime became a matter of considerable interest with the introduction of the basic oxygen converter steel furnace during the 1950s [1]. The use of quicklime instead of limestone and the rapid acceptance of this technology during the 1960s caused the definitive substitution of open-hearth steelmaking furnaces, and created parallel a revolution within the lime industry [2]. Nowadays, lime reactivity plays a critical role in different industrial processes. It is especially important to make a good slag facilitating the removal of sulphur and phosphorus and...
1 Introduction
The reactivity of commercial lime became a matter of considerable interest with the introduction of the basic oxygen converter steel furnace during the 1950s [1]. The use of quicklime instead of limestone and the rapid acceptance of this technology during the 1960s caused the definitive substitution of open-hearth steelmaking furnaces, and created parallel a revolution within the lime industry [2]. Nowadays, lime reactivity plays a critical role in different industrial processes. It is especially important to make a good slag facilitating the removal of sulphur and phosphorus and for providing a safer platform to withstand high-intensity arc plasma in the electric arc furnace, and violent reactions in the basic oxygen furnace [3]. Lime reactivity is also required as a fundamental process parameter in several industrial sectors including the constructions, chemical, mining, agricultural, geotechnical, pharmaceutical, environmental and alimentary sectors, i.e. for removing impurities, as a fluxing or leaching agent, as well as, for acid neutralization, pH stabilization and flue gas desulphurization [4]. Lime reactivity in the building lime sector is traditionally evaluated according to water slaking rate tests [5-6]. Pioneering studies on lime reactivity were performed during the 1970s under the auspices of the US National Lime Associations [7-8]. Since the beginning of the 2000s, several studies have pointed out the effect of particle size distribution, calcination kinetics, specific surface area, apparent density and impurity content [9-11]. Over the last ten years, an increasing number of mineralogical, petrographic and crystallographic studies have been performed, to bridge the geological gap. Geoscientists have proposed studies based on microfacies analysis, petrophysical properties and crystallographic characterization of carbonate rocks and derived burnt products [12-19].
Nowadays, Twin Shaft Regenerative (TSR) kilns, are generally considered the best technology to achieve the goal of soft-burnt reactive quicklime with different types of fuels [20]. In particular, TSR kilns present the lowest specific energy consumption compared with other types of kilns owing to the regenerative process [21]. Indeed, TSR kilns represent the best compromise for lowering costs, reducing gas emissions, and limiting environmental impact. According to Cimprogetti’s expertise, the production of soft-burnt quicklime in TSR kilns firing solid fuels, i.e. coal and petcoke, is a critical task.
Therefore, the main goal of this study was to investigate the burnability of different calcium carbonate rocks supplied in the typical crushed fractions, considering the range of temperature occurring in TSR kilns firing solid fuels. Rock samples with different geological origin, stratigraphic position, fabric and composition were supplied from producers worldwide. Multidisciplinary research was carried out to investigate heating behaviour, overburning tendency and quicklime reactivity. Moreover, the calcination kinetics were determined by means of thermal analysis on massive samples. Finally, the BET surface area and crystallographic parameters of the lime were considered, too. The statistical analysis allowed the evaluation of correlations between different data-sets and the slaking reactivity. Results put further constraints on the suitability of different carbonate rocks for lime manufacture in TSR kilns using solid fuels.
2 Tests and methods
2.1 Mineralogical-petrographic
and crystallographic analyses
The microfacies analysis of carbonate rocks according to their depositional textures and diagenetic modifications was performed in compliance with [22]. Crystal fabric analyses allow marble types and deformation styles to be distinguished, too. The identification of mineralogical phases was performed by means of X-ray powder diffraction analysis (XRD). Moreover, the quantitative phase analysis was carried out using the Rietveld method [23].
2.2 Burning trials, thermal analysis
and slaking tests
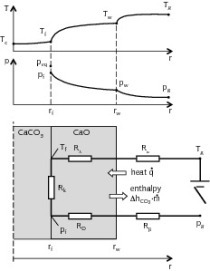
Burning trials were carried out on rock samples as received in an electric muffle furnace under air conditions. Burning temperatures between 1050 – 1300 °C were considered for simulation of the calcination process in TSR kilns using different kinds of fuels. The thermal analysis was performed on massive rocks in a thermogravimetric muffle furnace at 1200 °C. Calcination parameters were extrapolated according to the Fuoss-Salymer-Wilson method, as reported by [18]. The lime reactivity was evaluated with the European slaking test [6]. According to the practice commonly used by lime producers, “high reactivity” is when ΔT 40 °C or t60 < 3 min, “medium reactivity” when t60 is between 3 to 6 min, and “low reactivity” when t60 > 6 min.
2.3 BET specific surface area and real density
The porosity of burnt limes was investigated by means of nitrogen absorption using a gas sorpto-meter. Especially, the specific surface area and the volume of monolayer coverage were determined according to the BET theory. The analysis of real density was performed with helium pycnometry.
3 Results
3.1 Characterization of carbonate rocks
and burnt limes
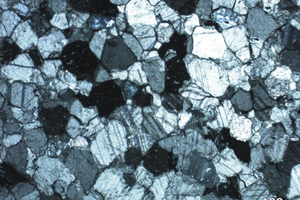
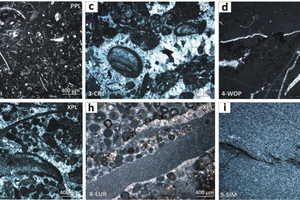
Preliminary geological information, origin, formation, lithology and fabrics are reported in Table 1. Selected samples, mostly high-calcium carbonates, cover a large part of the geological time-scale, ranging from the Neoarchean (2520 Ma) era up to the Eocene (34 Ma) epoch. The most significant rock fabrics are shown in Figure 1. The mineralogical analysis shows that some samples are slightly dolomitic in composition; some others are enriched in quartz and/or clay minerals (Table 2). Burnt limes from slightly dolomitic limestones typically present a significant content of periclase (MgO) associated with lime (CaO). Cementitious mineral phases, such as di-calcium silicate or larnite (Ca2SiO4), tri-calcium silicate or hatrurite (Ca3SiO5), aluminate phase (Na2xCa3-xAl2O6), and ferrite or brownmillerite (Ca2(Alx,Fe1-x)2O5) are present as associated constituents in moderately hydraulic limes from slightly impure carbonates. Moreover, limes from South African microbialites presented a significant content of di-calcium manganate Ca2(MnO4) [17]. Detailed chemical-physical, mineralogical-petrographic analyses of burnt limes are available in [19].
3.2 Thermal behaviour characterization
and slaking reactivity
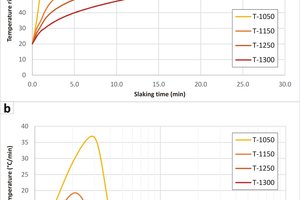
Results from thermogravimetric analysis showed significant differences in calcination rates. The highest (apparent) activation energy, calcination velocity and starting calcination time/temperature were observed for the coarse-sized granoblastic marble from Carrara (CAR). Conversely, the lowest (apparent) activation energy, calcination velocity and starting calcination time/temperature were observed for the micritic marly limestone from Verona (VER), respectively (Table 3). Results of the slaking tests, in terms of temperature rise, t60, and the maximum slaking temperature, Tmax, are reported in Table 4. An example of slaking rates of limes burnt at different T is reported in Figure 2. Soft-burnt limes at 1050 °C showed a t60 from 0.4 – 2.2 min; medium-burnt limes at 1150 °C showed a t60 from 0.5 – 10.9 min and, finally, dead-burnt limes at 1250 °C showed a t60 from 1.3 – 22.2 min. At the same time, the Tmax ranged between 82.7 and 70.5 °C for soft-burnt limes at 1050 °C, between 79.2 and 66.0 °C for medium-burnt limes at 1150 °C, and between 79.2 to 63.6 °C for dead-burnt limes at 1250 °C. The coralline limestone from Indonesia (PSP) presented the highest reactivity at 1050 °C (t60 = 0.3 min, Tmax = 85.5 °C), while the stromatoporoidal limestone from Sweden (SMA) presented the lowest reactivity at the same temperature (t60 = 2.2 min, Tmax = 70.6 °C). On the other hand, the fine-grained marble from Romania (SIM) presented the highest reactivity at 1250 °C (t60 = 1.3 min, Tmax = 79.2 °C), while the microbialite from South Africa (IDW) the lowest reactivity at the same temperature (t60 = 22.2 min, Tmax = 63.5 °C).
4 Discussion
4.1 Impact of rock fabric and impurity content
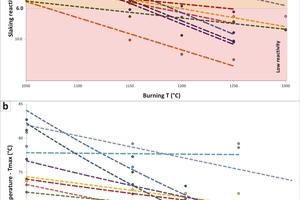
The burning temperature is one of the most important process parameters affecting lime slaking [4]. Effectively, the statistical analysis indicated the inverse correlation between the slaking reactivity and the burning temperature (Figure 3). Moreover, the deeper the slope of the slaking curves is, the higher is the overburning tendency at high temperature. Significant differences in slaking rates and overburning tendency can be firstly related to different rock fabrics and impurity content, i.e. different mineralogical-petrographic compositions. Based on this evidence, samples can be divided into four main groups, as follows:
1) The first group is represented by samples presenting very high reactivity at 1050 °C and very low reactivity at 1250 to 1300 °C. These samples showed the highest overburning tendency, testified by the deepest slope of the slaking curves in Figure 3. The samples are characterized by large calcitic fabrics, i.e. coralline limestone with mosaic calcite cement fillings (PSP) and coarse-grained marble (CAR), associated with very low impurity content
2) The second group is represented by samples presenting very high reactivity at 1050 °C and high- medium-high reactivity at 1250 to 1300 °C. These samples showed a low overburning tendency, testified by the gentle slope of the slaking curves in Figure 3. The samples are characterized by fine-grained calcite fabrics, i.e. fossiliferous packstone/grainstone associated with diagenetic microsparite (PRO) and fine-grained calcitic marble (SIM) with low impurity content
3) The third group is represented by one sample presenting medium-high reactivity at 1050 °C and very low reactivity at 1150 to 1300 °C. This sample showed a very high overburning tendency, testified by the deep slope of the slaking curves in Figure 3. This is the South African microbial boundstone (IDW) associated with medium-high impurity content
4) The fourth group is represented by samples presenting medium-high reactivity at 1050 °C and medium-low reactivity at 1250 to 1300 °C. These samples showed the lowest overburning tendency, testified by the gentle slope of the slaking curves in Figure 3. These samples are characterized by fine-grained fabrics, i.e. mainly mud-supported or micritic limestones (VER, CR1, WOP, SMA) associated with medium-high impurity content, i.e. quartz and clays scattered within the micritic matrix and sporadic diagenetic dolomite replacements
A summary of this classification is reported in Table 5. Limits between different groups are not strictly defined because of the large variability of geological or intrinsic rock parameters. Some important remarks can be derived, as well:
1) Medium- up to coarse-grained marbles, grain-supported and coralline limestones, characterized by large calcite cement fillings or replacements, are affected by the overburning tendency more than fine-grained marbles and/or mud-supported or micritic limestones. Lime particles produced by decomposition of coarser-grained rock fabrics appear more prone than finer-grained ones to coarsening and sintering. This fact is consistent with data from the thermal analysis, i.e. the kinetics parameters extrapolated from thermogravimetric analyses on massive samples. Moreover, it is in line with the pseudomorphic and topotactic calcination reactions reported by [13] and other studies on the effect of “limestone microstructure”, i.e. rock fabric, on the (apparent) activation energy [15, 17-18]
2) The impurity content is a key factor at T > 1250 °C, controlling the formation of different cementitious minerals, i.e. larnite, hatrurite, brownmillerite and aluminate phase [24]. Cementitious minerals slake at higher rates in respect of the sintered dead-burnt lime. Moreover, they form a boundary layer between lime crystallites, acting as sintering inhibitors at high temperature [10]. This is the reason why hydraulic limes showed lower overburning tendency than pure limes at high temperature.
4.2 Impact of quicklime physical parameters
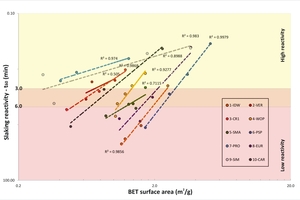
Derived physical-chemical, mineralogical and microstructural parameters also affect the quicklime reactivity [4]. Effectively, experimental data confirmed the significant impact of the BET surface area and the real density on slaking rates. The correlation between the slaking reactivity, i.e. t60, and the BET surface area is reported in Figure 4. Trends are consistent with expectations, but absolute values for the BET area are sometimes not comparable between samples of different origin. The Russian sample PRO, for instance, showed very high reactivity at 1150 to 1250 °C, which is apparently not congruent with very low values for the BET area. Conversely, other samples presenting lower slaking rates at the same temperature, exhibit higher values for BET area. Unexpected and substantially unexplained results have already been reported in the literature [25]. Moreover, the statistical analysis indicated the inverse correlation between the BET area and the real density based on gas pycnometry (Figure 5). This is consistent with the expectations and results reported in [18].
4.3 Analysis of lime crystallographic
and microstructural parameters
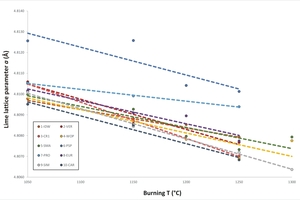
The lime (CaO) lattice parameter (crystal system, cubic) was plotted against the burning temperature and the slaking reactivity. A well-defined inverse correlation is observed between the Rietveld refined lime lattice parameter a (Å) and the burning temperature (°C) (Figure 6). General trends of lattice contraction at high temperature occur for all samples. This evidence has already been reported in the literature [26], but no clear explanation has been provided. We suggest that the observed contraction of the lime unit-cell can be interpreted taking into account the temperature-dependent miscibility gap between CaO and MgO. In fact, the incorporation of smaller Mg2+ ions (ionic radius 0.72 Å) replacing larger Ca2+ ions (ionic radius 1.00 Å) in the lime lattice is increased with increasing firing temperature, thus explaining the contraction. From the technological point of view, a larger amount of Mg incorporated in CaO would contribute to a decrease of reactivity owing to the low hydration kinetics of the periclase to form brucite, Mg(OH)2, at room pressure [4].
5 Conclusion
This study allowed the investigation of the burnability, overburning tendency, and lime slaking reactivity of carbonate rocks in the typical range of temperatures occurring in a TSR kiln firing solid fuels (1150 to 1300 °C). Crushed rock fractions were submitted to burning and slaking tests. A multidisciplinary analytical approach, including chemical-physical, mineralogical-petrographic, crystallographic and thermal analyses, was carried out on raw materials and burnt products.
The first part of the study allowed identification of the rock fabric and the impurity content, i.e. the mineralogical-petrographic composition, as key factors controlling the slaking rates and the overburning tendency of the lime at high temperature. Especially, coarse-sized granoblastic marbles, grain-supported and coralline limestones, presenting calcite cement fillings and/or replacements, are affected by higher overburning tendency than fine-grained marbles and/or mud-supported or micritic limestones. Moreover, hydraulic limes showed higher reactivity than pure limes at high temperature. This fact has to be related to the formation of different cementitious minerals at T > 1250 °C, which slake at higher rates than the dead-burnt lime. Moreover, they could act as sintering inhibitors for the lime at high temperature [10]. Results from calcination kinetics analysis performed on massive samples allowed the conclusion that the higher the grain/crystal sizes of carbonate rocks are, the higher are the (apparent) activation energy, the calcination velocity, the starting calcination time/temperature and the slaking reactivity. This is consistent with a previous study performed on massive granulated samples reported by [18], and pseudomorphic and topotactic calcination reactions reported by [13].
The second part of the study allowed identification of the impact of lime physical-chemical and mineralogical-crystallographic parameters on slaking reactivity. The statistical analysis indicated the direct correlation between BET surface area and the temperature rise, i.e. t60, which is also consistent with the lime densification at high temperature. The contraction of the lime unit-cell as a function of temperature and bulk Mg content is readily explained by the higher incorporation of Mg in the CaO lattice. The higher miscibility of MgO with CaO crystal structure at 1300 °C is also consistent with a lower slaking reactivity of the lime product, considering that the hydration kinetics of periclase at room pressure is extremely low.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.