Hydrophobizing of gypsum by silanes (Part 1)
The main disadvantage of gypsum products in the building industry is the poor water resistance. While it is known that the water resistance can be increased by adding special silanes, only very little is known about the working mechanism of such silanes. The mechanism of the hydrophobizing effect of propyltriethoxysilane as a representative for a short chain alkylalkoxysilane in an alkaline gypsum matrix is studied in detail.
1 Introduction
1 Introduction
With the present study the effect of alkyltriethoxysilanes: Si(OCH2CH3)3CnH2n+1, one group of silanes, using the example of propyltriethoxysilane (PTES, n = 3) is investigated. PTES was chosen based on our first indications that the hydrophobizing effect clearly depends on the alkyl chain length [9]. Thus, short alkyl chain lengths alkylalkoxysilanes (n < 5) are principally suited to increase the water resistance of alkalized cured gypsum formulations. The shorter the alkyl chain length of the alkyltriethoxysilane the lower the resulting water uptake of the gypsum product. The lowest water uptake was measured for methyltriethoxysilane. With octyltriethoxysilane water uptake is in the same order of magnitude as without hydrophobizing additive [9].
As generally known, alkyltriethoxysilanes react in presence of water to silanols and afterwards to siloxanes. Both reactions can be catalyzed acidic or alkaline. For gypsum products the alkaline catalysis is of more interest, since some products are already alkalized such as gypsum lime plasters. The hydrolysis reaction of propyltriethoxysilane (n = 3) starts according to reaction 1 by replacement of one ethoxy-group by a hydroxy-group. This step is a nucleophilic substitution according to SN2 [10] (R = Propyl).
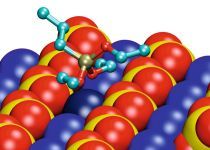
Based on these indications, detailed investigation using gypsum surface hydrophobicity tests, NMR measurements (Part 1) and adsorption simulations (molecular dynamic) (Part 2) were carried out to gain a deeper insight into the mechanism of the hydrophobizing effect of propyltriethoxysilane (PTES) as an example for a short-chain alkyltriethoxysilane.
2 Materials and methods
For detection of silane species by 29Si-NMR-spectroscopy concentrations higher than 0.5 wt% in an aqueous solution were necessary. Since propyltriethoxysilane is not miscible with water it was mixed 1:1 with ethanol. To catalyze the hydrolysis reaction with low pH as well as with high pH a few drops of hydrochloric acid (pH 1) and sodium hydroxide solution (pH 14) respectively, were added to 3 mL silane-ethanol mixture with a resulting pH-value of 3 and 12.5 (pH electrode, type ROSS, Orion).
All NMR-spectra were recorded with the DPX 400 from Bruker. The resonance frequencies of the nuclei were 100.6 MHz for 13C and 79.49 MHz for 29Si. As insert deuterized benzene was added together with the standard tetramethylsilane (TMS). The spectra were analyzed with the software Topspin 2.1 from Bruker.
3 Results and discussion
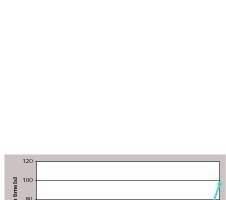
A significantly reduced water uptake is achieved with increasing content of propyltriethoxysilane even though only a small percentage range was tested. Investigations with significantly larger amounts of PTES than 1.33 wt% were not taken into account, since any higher concentrations become uneconomical in industrial applications.
When using the alkyltriethoxysilane OTES no reduced water uptake could be observed. The time of the water drop absorption was identical to the gypsum surface prepared without OTES in the formulation according Aberle et al [9]. Thus, OTES was no longer considered in the following experiments.
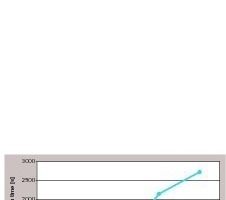
Subsequently, the hydrophobizing effect of the PTES was investigated with a fixed content of 0.165 wt% as a function of the workability time of the fresh gypsum mortar. A variation of the workability time was realized by the use of a-hemihydrate with different ratios of b-hemihydrate, because the workability time of a fresh gypsum mortar of a-hemihydrate is longer compared to the one of b-hemihydrate. Use of retarding or accelerating agents was avoided to eliminate their possible influence with respect to the silane effect. Workability time of pure a-hemihydrate (with 0.5 wt% Ca(OH)2) was reduced from 15 minutes to a few seconds by successive addition of b-hemihydrate. By the subsequent water drop test an increased hydrophobicity was found with increasing time of workability (Fig. 3).
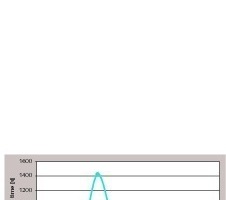
This result led to the assumption that the hydrolysis time of PTES and the maximum hydrophobicity of the treated gypsum are correlated. In order to verify the assumption, the hydrolysis time was increased by a pre-hydrolysis reaction of PTES between 5 minutes and 60 minutes in a Ca(OH)2-suspension (with 0,5 wt% Ca(OH)2 with respect to the hemihydrate powder). The suspension was used as mixing water after the different times of pre-hydrolysis. In this way a total hydrolysis time window up to 75 min (60 minutes pre-hydrolysis time + 15 minutes workability time of fresh mortar, pure a-hemihydrate was used) could be scaled. As can be seen in Figure 4, at a hydrolysis time of 35 minutes there is a maximum of the hydrophobizing effect (PTES content always 0.165 wt%). Afterwards the hydrophobizing effect decreases again. According to the 29Si-NMR spectra condensation products occur in the time scale between 30 and 45 minutes – probably, the reason for a reduction of the hydrophobizing effect.
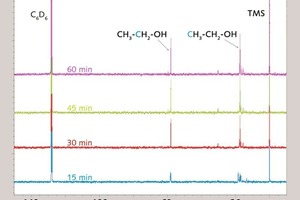
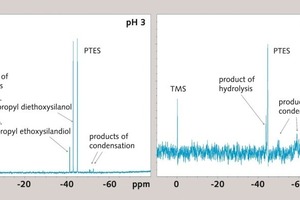
Figure 6 shows the 29Si-NMR-spectrum of PTES at pH 12.5 after 35 minutes hydrolysis time. Only one of three possible hydrolysis products (-ol, -diol, -triol) was detected. From the 29Si-NMR-spectra of tetraethoxysilane, TEOS [20] it can be concluded, that the formed hydrolysis product is propyldiethoxysilanol (hydrolysis product of reaction 1). The signals of further hydrolysis products occur at higher chemical shifts to the signal of the initial silane. In the PTES-spectrum (Fig. 6, right) signals of condensation products appear on the right-hand side of the initial PTES-signal after 35 minutes.
The measurement was repeated at pH 3 to take a closer look at the hydrolysis of PTES. According to Fig. 1 [14, 19] the hydrolysis reaction at acidic pH value should be faster compared to the condensation reaction. As can be seen in the spectrum (Fig. 6, left) two of three possible signals of hydrolysis products occur after 35 minutes at pH 3. These are propyldiethoxysilanol and propylethoxysilandiol. Therefore the occurring hydrolysis product at pH 12.5 is propyldiethoxysilanol (lowest shift to PTES, the –diol and –triol appear with higher shift to PTES). The difference of the chemical shift between the spectrum at the acidic and of the basic pH value can be explained with the deprotonation of the silanol molecules at pH 12.5 [21].
As a conclusion, hydrolysis species like propyldiethoxysilanol seem to cause the hydrophobizing effect of alkalized gypsum, especially in the case of an optimal workability time scale of about 35 minutes. Is this crucial period exceeded due to a too long hydrolysis time, siloxane condensation products form in the aqueous solution to such an extent that the treated gypsum is insufficiently hydrophobized.
In Part 2 (ZKG 9/2013), the molecular simulations (molecular dynamics) follow. With their help the interactions of the silanes with crystalline gypsum surfaces at the molecular level are described and analyzed. The results will be evaluated and summarized together with the experimental results so far obtained.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.

![1 Relative reaction rate vrel for hydrolysis and condensation reaction of silanes as a function of pH [15, 20]](https://www.zkg-online.info/imgs/tok_c87ce0002aa8d6b55597e6ef584b661b/w300_h200_x400_y306_101535921_9c0bda7409.jpg)