Can mixtures of a- and b-hemihydrates be quantified by means of thermoanalysis?
Summary: a-hemihydrate is mainly used for the manufacture of high-quality products of gypsum building material. The b-type is used for the production of articles made in bulk. The question as to what extent mixtures of a- and b-hemihydrate will already result from manufacturing processes is still open, because there is no suitable detection technique. Up to now thermal analysis has been taken into consideration for the quantification, since a- and b-CaSO4 ∙ 0.5 H2O differ due to an exothermic effect at different temperatures. Depending on the crystal shape and size, however, this effect cannot be measured uniformly. The reason is a kinetics of the phase transition with increasing temperature that is depending on the crystallite form. Therefore, thermal analysis is excluded for the quantitative analysis of a-/b-mixtures.
1 Introduction
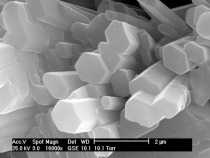
Calcium sulphate-hemihydrate (CaSO4 ∙ 0.5 H2O) is manufactured from gypsum (CaSO4 ∙ 2 H2O) by partial dewatering in two different ways. In the presence of high partial water vapour pressures at temperatures between 80 °C and 120 °C (hydrothermally in a steam autoclave), but also in acids or salt solutions at temperatures above 45 °C, the hemihydrate is formed as well-developed hexagonal prisms (Fig. 1a). At low partial water vapour pressures, e. g. during the dewatering of gypsum in air or in a vacuum at temperatures between 45 °C and 200 °C, the so-called beta-form of the...
1 Introduction
Calcium sulphate-hemihydrate (CaSO4 ∙ 0.5 H2O) is manufactured from gypsum (CaSO4 ∙ 2 H2O) by partial dewatering in two different ways. In the presence of high partial water vapour pressures at temperatures between 80 °C and 120 °C (hydrothermally in a steam autoclave), but also in acids or salt solutions at temperatures above 45 °C, the hemihydrate is formed as well-developed hexagonal prisms (Fig. 1a). At low partial water vapour pressures, e. g. during the dewatering of gypsum in air or in a vacuum at temperatures between 45 °C and 200 °C, the so-called beta-form of the hemihydrate (plaster), is formed. This has a strongly fissured structure that still possesses the external form of the original dihydrate (Fig. 1b).
The different surface characteristics of the two forms are reflected i.a. in their setting properties. The formation of dihydrate from the a-form takes place significantly more slowly than in the case of the beta-form, which results in the achievement of higher strength values. The a-hemihydrate is therefore employed for the production of high-quality special binding agents, such as plaster for moulding, grouting for ceramic materials, trowelling compounds, self-levelling floor screeds or whiskers. Large quantities of b-hemihydrates are used for the production of sandwich-type gypsum plaster boards and gypsum wall panels. For some product grades, mixtures of the two forms are employed. There is also an interest in quantifying mixtures of the two forms, as such mixtures can also result from the manufacturing process. The hemihydrate forms are therefore frequently the subject of investigations with regard to their physical and chemical properties.
2 Crystalline forms of a-CaSO4 ∙ 0.5 H2O
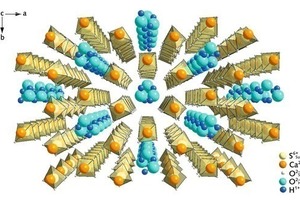
Additives for influencing the crystalline form, such as carbonic acids and their salts, polymers and inorganic substances, are used in the autoclave process for a-hemihydrate production in order to favourably influence the subsequent stages of product processing. The resulting forms can range from filamentous or rod-shaped to discoid crystallites (Fig. 2). Between the first and last-named morphology a shortening of the crystallographic c-axis takes place, which is also associated with a shortening of the “water channels”. Figure 3 shows the hemihydrate structure with corresponding direction of view along the “water channel” arrangement and along the crystallographic c-axis.
3 Thermal characteristics
Depending on the partial pressure of the water vapour, CaSO4 ∙ 0.5 H2O dehydrates at temperatures between 100 °C and 200 °C. At a normal low vapour pressure, such as occurs in an open crucible with dry gas stream during a TG/DTA measurement, the loss of water begins at 100 °C and concludes at between 120 °C and 140 °C, depending on the quantity of material and the rate of heating. During this process the water molecules can leave the structure (Fig. 3) without hindrance and the dry structure thereby created is the soluble anhydrite (A III). This metastable phase transforms in a kinetically controlled exothermic reaction into the stable anhydrite (A II). This transformation takes place in two different modes for the a- and b-forms. For b-hemihydrate, a broad exothermic effect is observed at 350 °C – 375 °C [2-4]. The occurrence of an exothermic effect for the a-hemihydrate at lower temperatures directly after the dewatering process is not consistently discussed in the literature. BUDNIKOV [5] considers that no exothermic effect is discernible in the thermogram of the a-hemihydrate. In contrast, KUNTZE [3] states that there is such an effect
at temperatures below 250 °C. According to [2] and [4] an exothermic effect occurs between 163 °C and 255 °C as a function of the partial water vapour pressure. Moreover, POWELL [2] noted exothermic effects of differing extents
in different samples with an identical quantity of analysis material.
TG/DTA analyses of a-hemihydrate samples (Fig. 4), as depicted in Figure 2, demonstrate that only the sample of rod-shaped crystallites displays an exothermic effect subsequent to the dewatering. The filamentous and discoid hemihydrates show no such effect. In the case of discoid hemihydrates, the dewatering itself commences at 90 °C and is concluded at 125 °C. The filamentous and rod-shaped crystallites lose their hydration water at temperatures above 100 °C. In the case of filamentous hemihydrates, the last water molecules do not leave the structure until a temperature of 160 °C, which is due to the long distance the H2O molecules have to travel along the filament (crystallographic c-axis) before they can escape. By means of in-situ Raman spectroscopy, the phase content of the a-hemihydrate forms was studied under conditions of corresponding temperature increase in order to obtain an explanation for the lack of exothermic effect in the case of filamentous and discoid hemihydrates. X-ray diffractometric measurements are not suitable for this purpose, due to the identical reflex locations of hemihydrate and A III.
The thermal characteristics of b-hemihydrate are depicted in Figure 5. As an example, two b-hemihydrates derived from source gypsums of different origin were measured. The known difference from a-hemihydrates is displayed in the significantly higher temperature range of the A III – A II transformation (between 350 °C and 400 °C). The corresponding exothermic effect varies in extent depending on the origin of the source gypsum and the process technology of the preparation.
4 In situ Raman spectroscopy
Using Raman spectroscopy (Fig. 6), CaSO4 phases can be identified by means of the molecule vibrations of the sulphate ion. In a Specac 21525 temperature cell, which can be adapted for use with the RFS 100/S Raman spectrometer and Nd:YAG laser from Messrs. Bruker, the conversion of a-hemihydrate crystalline forms was studied under cell temperatures of up to 220 °C on the basis of change in sulphate bands and compared to that of a b-hemihydrate. Figure 7 shows the respective sections of the Raman spectra of the a-hemihydrate forms for the symmetrical stretching vibration (ns). In the case of the filamentous hemihydrate, not only does the ns band of CaSO4 ∙ 0.5 H2O occur at 1015 cm-1 at temperatures above 95 °C, but also the ns band of the A III occurs at 1026 cm-1 and gains in intensity with increasing temperature. Above 165 °C, the ns band of the A II appears as a bump at 1017 cm-1, but then decreases in intensity as the temperature rises to 220 °C. Up to this temperature, the main phase remains the A III. The phase formation of the discoid hemihydrate is similar. The A III band is prominent at 110°C adjacent to that of the hemihydrate. Formation of A II commences at 165 °C. In this case, too, the A III persists up to 220 °C alongside the A II, which forms only slightly faster than in the case of the filamentous form. The situation is different in the case of the discoid form, in which the A II formation does not commence until above 180 °C and at 220 °C already represents the major portion alongside A III. The temperature-time interval of the A III conversion is significantly smaller in this case, and the TG/DTA measurement therefore reveals this to be an exothermic effect.
The collapse of the dry skeletal structure of the hemihydrate (A III) is apparently strongly dependent on the surface characteristics of the original hemihydrate phase. As a result, the transformation of A III into A II can only proceed slowly over a broad temperature range and the released heat is consequently extremely difficult to measure. However, if this transformation takes place within a short period, a clear thermal effect can be detected. Such differences could also be caused by mechanical stressing, such as that resulting from grinding of the CaSO4 ∙ 0.5 H2O crystallites [6].
In the case of b-hemihydrate, only the formation of A III could be observed up to 220 °C, which is consistent with the TG/DTA curve (Fig. 5). The Specac cell with its maximum temperature of 220 °C is not designed for measurements up to the transformation temperature of A III into A II.
5 Conclusion
The question of a quantitative test procedure for mixtures of a-/bhemihydrates is still open. As is generally known, the two forms of CaSO4 ∙ 0.5 H2O are not distinguishable by means of X-ray-photography. The thermal analysis taken into account so far is also excluded as quantifying method. Certainly both forms differ due to the occurrence of an exothermic effect at different temperatures, but this effect cannot be measured uniformly depending on the crystal habit and size.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.