Determination of hardened binder initial composition
A new method based on the carbon isotope 14C/12C ratio determination can be used for the characterization of binder contents in hardened structures.
1 Introduction
1 Introduction
Cement-based materials hold by far the largest market share. However, the setting and hardening of cement-based materials is too complex for an initial testing of a hypothesis, i.e. if the carbon isotope ratio of CaCO3 can be used for the determination of the hardened cement mortar initial composition. As a result, in this work we are concerned with lime-based materials as a simplified model system.
Examples of the use of the carbon (and oxygen) isotope ratio of the lime mortars can be found [1-3], but mostly in the context of radiocarbon dating of ancient mortars. The difficulty of accurate, unbiased dating of old lime binders comes mainly from the presence of inclusions of unburned char in the original lime and the inherent difficulty of distinguishing between the calcium carbonate used as aggregate and that formed by lime setting.
Lanas and Alvarez [4] and especially Lawrence et al. [5] used thermogravimetric analysis (TGA) for determination of the quantity of unreacted hydrated lime and calcium carbonate in lime mortar samples. TGA analysis of lime samples is widespread and relies on more or less well separated processes of mass loss of water from portlandite and CO2 from CaCO3.
Standard methods for determination of the composition of binders taken from old/hardened structures are not very precise (ASTM C-1084-97) and the proposed research aims to contribute to the current methods of hardened binder composition determination. To the best of our knowledge, the method of carbon isotope 14C assisted determination of the initial composition of hardened binder is new and is therefore tested against the simplest inorganic binder i.e. lime mortar. The application of the same methodology for the analysis of hardened cement-based materials is in progress.
2 Theoretical background
3 Experimental part
Physical and chemical characteristics of the hydrated lime used for the preparation of the test specimens are shown in Table 2. The physical and chemical characteristics of the aggregate used for preparation of the test specimens are shown in Table 3. The test samples were mixed in a mixer in accordance with HRN EN 196-1. Environmental conditions in which the samples were mixed and stored until testing were 20 °C±2 °C and minimum 50 % RH. The lime mortar samples aged for about 150 days were subjected to 14C analysis. A sample taken for the 14C analysis was treated with diluted hydrochloric acid in order to decompose CaCO3. The CO2 captured is further chemically converted into benzene, C6H6, in a form suitable for measurement. Samples were measured by liquid scintillation counting (Fig. 1; LSC Quantulus 1220, PerkinElmer) [9].
Radiocarbon activity of the samples was expressed as a relative measure of modern carbon activity, pMC. Oxalic acid was used as the active standard, while anthracite and marble were used as background samples and the results were corrected for isotopic fractionation [10]. This is the usual way of expressing the results of 14C activity measurements, and has the following simple interpretation. Determining a 0 pMC activity would mean that all the CO2 captured (liberated from the sample under investigation) is so old that it does not have any measurable activity. On the other hand, determining a 100 pMC activity would mean that all the CO2 captured (liberated from the sample under investigation) comes from the present day, the modern CO2 which we breathe. Aggregate has effectively zero pMC activity because it is older than 50 000 years. It does not contribute to the 14C activity, instead it dilutes the activity of the CO2 captured by lime hydration (Eq. 1).
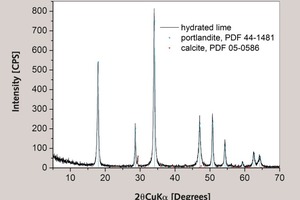
The composition of the lime mortar samples and materials used was investigated by powder X-ray diffraction (XRD). A Shimadzu H-6000 diffractometer with Cu Ka radiation was used (the scan step was 0.02 ° with an integration time of 1 s). The hydration was blocked and free water removed by the addition of acetone (2‑propanon). This was done by grinding the sample with three doses of acetone in agate mortar. TGA analysis of lime mortars was conducted on a Perkin Elmer TGS-2 thermal analysis system at a heating rate of 10 K/min and a synthetic air purge flow of 15 cm3/min.
4 Results and discussion
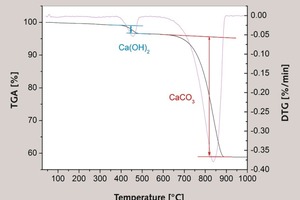
Lime mortars contain some unreacted Ca(OH)2 since the carbonation reaction (Eq. 1) slows down as the unreacted Ca(OH)2 is more and more hindered by the low permeability layer of the previously formed CaCO3. The lime mortars typically show two main processes accompanied with weight loss. The first process corresponds to the chemical decomposition of portlandite in a 380–500 °C temperature interval, while the second process corresponds to the CaCO3 decomposition and occurs between 580 and 910 °C (Eqs. 2 and 3, respectively).
The calculation of initial lime mortar composition (weight percent of portlandite (binder) to calcite (aggregate) mass ratio) presented in Table 4 is as follows:
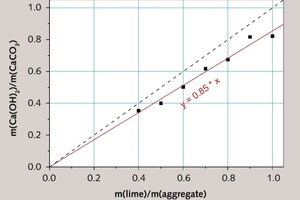
The typical result of TGA analysis of lime mortar is shown in Figure 2. In the same figure, the result of DTG (first derivative of mass loss curve) is also shown and two well separated processes of mass loss could be observed, which is in accordance with other investigators [4-6]. Comparison of the initial lime mortar composition determined from the TGA results corrected for 14C isotope activity (Table 4) to the known lime mortar composition (Table 1) is shown in Figure 3. It is possible to account for 85 % of the “true” binder to aggregate, i.e. lime to limestone aggregate ratio of the investigated lime mortars. There are several possible reasons for the underestimate.
If the CaCO3 present in lime used for lime mortar sample preparation comes from unburned limestone, it decreases the 14C carbon isotope activity while increasing the quantity of CaCO3 determined by TGA. If we assume 99 % pure Ca(OH)2 containing only 1 % calcite, then for a composition rich on lime mortar such as sample #7, it results in 495 g of Ca(OH)2 and 505 g of CaCO3, changing the Ca(OH)2 to CaCO3 ratio by as much as -2 %. If, on the other hand, the CaCO3 present in lime used for lime mortar sample preparation comes from partially carbonated lime, then the ideal pMC activity decreases by about 1.6 % for 10 % by weight CaCO3 in partially carbonated lime. The calculated initial Ca(OH)2 to CaCO3 weight ratio decreases by about 2.6 %. Results of XRD analysis of the lime used for experiments confirm that it is mainly composed of portlandite (Ca(OH)2) with some calcite (CaCO3) present, as suggested by chemical analysis of the raw material.
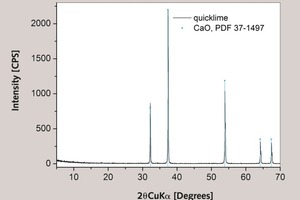
The influence of the presence of periclase, MgO, or brucite, Mg(OH)2, in the lime is not taken into account, since they were not detected (by TGA or XRD) either in quicklime (Fig. 5) or the slaked lime produced from it. For reasons of simplicity, MgO could be regarded as “inert impurity” together with other oxides, Fe2O3, Al2O3, SiO2, alkalis and SO3 determined by chemical analysis. By simply assigning the actual weight percent of portlandite in slaked lime by p, and the actual weight percent of CaCO3 in aggregate by c, then it follows that one (ideally) actually finds the true Ca(OH)2 to CaCO3 ratio. The initial lime to aggregate mass ratio could be obtained if actual purities are known, and experimentally found m(Ca(OH)2)/m(CaCO3) multiplied by c/p. In a case such as ours, where limestone is of greater purity than the quicklime obtained from it, it is likely that the experimentally found initial lime to aggregate mass ratio in lime mortar is underestimated by several percent. The presence of organic impurities or additives in the investigated samples should not contribute to the measured pMC activity, since the CO2 obtained from the sample is not obtained by sample burning but with acid digestion.
Carbon isotope fractionation in lime mortars had been studied by Pachiaudi et al. [3]. The observed carbon isotope fractionation differed for calcareous and quartz aggregate samples, and for the sample that had numerous cracks. Although isotope fractionation in the course of diffusion of carbon containing species through the porous lime mortar is theoretically possible. Due to the lack of other studies, we could not currently determine the extent of its significance, or if it is significant at all (due to the precision of 14C activity determination). It is likely that even if there is a measurable effect of isotopic fractionation, it also depends on the sample cracking [3]. 14C/12C carbon isotopic ratio of lime mortar could also change due to the periodic wetting and drying (rain containing dissolved aggressive CO2). Another noteworthy detail is the seasonal 14C/12C carbon isotopic ratio change. Recent work by Southon [11] raises questions about isotopic fractionation corrections. The pMC activity could also change significantly if the lime carbonation had been deliberately accelerated by burning of fossil fuel, thus enriching the air with 12CO2.
5 Conclusions
Acknowledgments

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.