Dispersion powders in cementitious systems
Depending on the saponification stability of the particular dispersion powder and its additive system the dispersion powders and their organic decomposition products can not only affect the mineral phase characteristics but may also have an elution behaviour that depends on the cementitious system and its pH.
Introduction
Introduction
The continuously advancing technical development of construction chemistry products, such as plasters, levelling mortars and thin-bed mortars, means that dispersion powders are a necessary constituent of the mix formulation. In the formulation of thin-bed mortars the dispersion powders raise the ductility of the cementitious binder matrix and form an adhesive bond to the reverse side of the ceramic unit due to their film-forming properties. In this case the adhesion to the ceramic construction product is determined not only by the physical and chemical parameters of the ceramic material, and in particular here by the reverse side of the ceramic unit and its boundary surface, but also by the resistance of the dispersion powder to the cementitious binder matrix. Cementitious thin-bed mortars are reactive binders so they are responsible for the durability of the entire system with respect to hardness and strength.
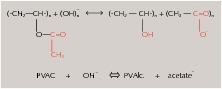
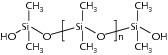
The high pH, especially of alkali-rich Portland cement binders, mainly affects the structure and composition of the polymers used. With long-term high alkalinity a saponification reaction may occur in the polymer and in the additive system (e.g. polyvinyl alcohol as a protective colloid).
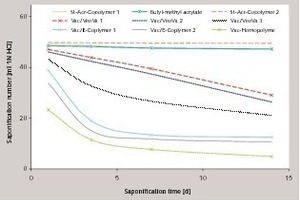
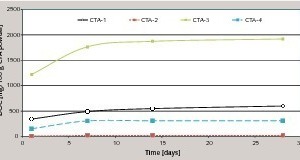
The investigation described here was used to determine the stability of dispersion powders present in a cementitious matrix. The saponification stability of the dispersion powder without a cementitious matrix was determined beforehand by determining the saponification number on dispersion powders before they were used in cementitious model mortars with different pH values. It was established that vinyl acetate homopolymers with a saponification number < 20 saponify extremely quickly with the formation of, among other things, water-soluble acetates. Vinyl acetate-versatate copolymers exhibit a higher stability, which was demonstrated with the aid of the eluted fractions of DOC (dissolved organic carbon). It was possible to verify high levels of stability to alkaline pH values, especially with acrylate and copolymers based on styrene acrylate (Fig. 1).
Materials and methods
The high pH values in Portland cement systems can cause saponification reactions in the polymer chains and in the additive system (protective colloid system). The main decomposition product in this case is water-soluble acetate. This has an effect on mineral phases that has already been investigated by M. Schmidt and H. Pöllmann in 2008 [2, 3] and by H. Pöllmann in 1989 [4, 5]. Decomposition reactions due to high alkalinity are also known for acrylates, versatates and other polymers (Fig. 2).
Commercial mortars as well as model mortars were employed in this investigation. The compositions of the model mortars are shown in Table 1.
Determination of the saponification number
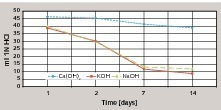
The dispersion powder to be investigated is redispersed in de-ionized water to form a dispersion containing 50 % solids. The redispersion is then neutralized with 1N sodium hydroxide and 1N hydrochloric acid to a pH of 7. Dispersion powders containing calcium carbonate (anti-caking agent) are dissolved by the addition of hydrochloric acid and are then no longer available to the back-titration with hydrochloric acid. The quantity of sodium hydroxide or hydrochloric acid added for the neutralization is taken into account in the weight of the sample. The calculated quantity of dispersion (pH 7.0) that contains 5 g of the solid is weighed out into a 100 ml screw-top glass jar with an accuracy of 0.01 g. 50 ml 1N sodium hydroxide are added to this with a pipette. The screw-top glass is closed immediately, the sample is homogenized by shaking strongly 20 times and is then stored at 50 °C. After appropriate time intervals (1 day, 3 days, 7 days and 14 days) the sample is cooled to room temperature and poured into a 250 ml glass beaker. Back-titration with 1N hydrochloric acid to pH 7 is then carried out on a magnetic stirrer using a pH electrode. The saponification number is given directly by the millilitres of 1N hydrochloric acid required during the titration. If 50 ml of 1N HCl is needed for the back-titration then this corresponds to a saponification number of 50, i.e. no saponification reaction has taken place. If the 50 ml of 1N NaOH is completely consumed in the saponification reaction then 0 ml hydrochloric acid is required for the back-titration, giving a saponification number of 0. Table 2 gives some of the saponification numbers determined for the respective types of polymer. The use of calcium hydroxide solution instead of KOH or NaOH indicated less saponification during the observation period (Fig. 3).
The DOC value was determined using an elution test set up in-house based on the column test in DIN 19528 [6] and the shaking test in DIN 19529 [7]. During the determination of the DOC value the quantity of organic constituents from dispersion powders was determined through the saponification products in the eluate.
Production of the test pieces
In method A (without water change) 50 ml of the eluate was taken each time, stabilized with 1 ml concentrated nitric acid, closed air-tight and analyzed for DOC value (using the method in DIN EN 1484 [9]). The 50 ml eluate that had been taken was replaced by fresh water.
In method B all the 750 ml of water were removed, the test piece was carefully rinsed under flowing water and then placed in 750 ml of fresh water again.
Results
On the other hand, styrene acrylates and pure acrylate dispersion powders exhibit a significantly lower sensitivity to high pH values. In some formulations containing vinyl acetate-versatate and other copolymers the content of protective colloid or the structure of the side groups appears to be responsible for different saponification numbers.
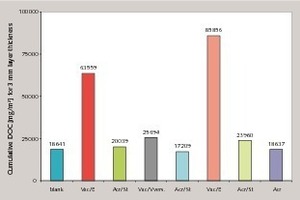
The use of calcium hydroxide as the saponification agent, such as occurs due to the hydration in binder systems based on Portland cement, also leads to saponification of the dispersion powder and therefore resembles real systems (Fig. 3) [8]. The saponification numbers given in Table 2 were taken as an indication of potential saponification in alkaline binder systems. Figure 4 shows the cumulative DOC values of different dispersion powders in an OPC system after elution for 875 days. It is clear that dispersion powders with low alkali sensitivities also form low DOC values in the eluate and dispersion powders with high alkali sensitivities exhibit high DOC values in the eluate. The saponification number measured by the given method therefore allows a direct qualitative conclusion to be drawn about the expected DOC value in the eluate. The extent of the DOC value in the eluate is governed quantitatively by the alkalinity of the binder system.
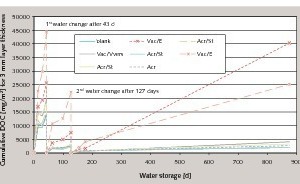
It was also established that different elution scenarios (method A without water change and method B with water change) lead to different DOC values. Figure 5 shows a comparison of the cumulative DOC values of different commercial mortars. In the elution scenarios following method A without water change, such as in swimming pools, there can be a continuous asymptotic rise in the DOC value (Fig. 5). In the elution scenarios following method B with periodic inflow and discharge of fresh water, as in drained systems, no saturation behaviour was detected within the measuring period of 875 days, so complete elution of water-soluble decomposition products can be assumed (Fig. 6). The quantity and duration is governed here by the pH value as well as by the elution scenario and the composition of the dispersion powder.
Conclusions
The use of calcium hydroxide as the saponification agent showed a reduced saponification reaction during the investigative period when compared with the higher pH values of sodium and potassium hydroxides.
It can therefore be assumed that a saponification reaction of the above-mentioned dispersion powders will also occur continuously in alkaline Portland cement systems, such as thin-bed mortars or plasters.
Depending on the elution scenario, different DOC values may be detected in the eluate according to the saponification state of the dispersion powder. The saponification number of the dispersion powder provides good qualitative information about the DOC value in the eluate.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.