Resistance of polymer modified calcium sulfoaluminate cement to sulphate attack
The effects of different dosages of EVA and SBR on the resistance of CSA paste to sulfate erosion under the condition of full soaking in sodium sulfate solution were studied. Based on the development rule of the flexural strength of polymer-modified CSA paste after immersion in Na2SO4 solution, combined with the flexural erosion coefficient, the resistance to sulfate erosion of polymer modified CSA paste was analyzed and evaluated. To reveal the mechanism, the effect of EVA and SBR on hydration characteristics and microstructure of CSA paste under different conditions were investigated by XRD, FTIR and SEM-EDS. Results show that the sulfate resistance coefficient increases gradually with the increase of EVA content; after adding SBR, its flexural erosion coefficient first decreases and then increases gradually with the increase of SBR content. Microscopic analysis shows that under the erosion conditions of the Na2SO4 solution, the amount of AFt in CSA paste decreases with the increase of EVA content, and first decreases and then increases with the rise of SBR content.
1 Introduction
A building structure is easily damaged if it is in the erosion environment for a long time. If the damaged area is not repaired in time, the damage will further develop, which will eventually lead to the scrapping of the building and may even cause safety accidents [1]. Polymer is a kind of repair material modifier with superior performance [2], and its incorporation can improve the erosion resistance, bonding and impermeability of cement mortar. Ethylene-vingyl acetate (EVA) and styrene-butadiene rubber (SBR) are widely used polymer materials because of their low cost, non-toxic...
1 Introduction
A building structure is easily damaged if it is in the erosion environment for a long time. If the damaged area is not repaired in time, the damage will further develop, which will eventually lead to the scrapping of the building and may even cause safety accidents [1]. Polymer is a kind of repair material modifier with superior performance [2], and its incorporation can improve the erosion resistance, bonding and impermeability of cement mortar. Ethylene-vingyl acetate (EVA) and styrene-butadiene rubber (SBR) are widely used polymer materials because of their low cost, non-toxic and harmless nature, and low pollution [3, 4]. At present, most studies on cement-based materials modified by EVA and SBR focus on Portland cement. However, due to the weak erosion resistance of Portland cement after hydration, it is difficult to meet the requirements of some repair projects for high durability with general polymer-modified cement [5-7]. Therefore, the application of traditional EVA and SBR modified mortar in repair engineering is limited. Calcium sulfoaluminate cement (CSA) has excellent characteristics such as anti-freezing, anti-permeability, anti-corrosion and low alkaline, and has considerable development and application prospects [8, 9]. However, there are few researches on the erosion resistance of EVA and SBR modified CSA-based materials. In order to expand its application field, it is very important to study the corrosion resistance and mechanism analysis of polymer-modified CSA-based repair materials.
Sulfate erosion is one of the important reasons for the damage of building structures, which seriously affects the durability of civil engineering, so it is very urgent to study the sulfate erosion of cement-based materials [10, 11]. Based on this, the flexural strength of polymer-modified CSA pastes fully immersed in 5% Na2SO4 solution at (20±1) °C was determined in this paper, and the sulfate erosion resistance coefficient of EVA and SBR modified CSA sample was obtained, then the effects mechanism of EVA and SBR on sulfate resistance of CSA pastes were studied by employing the characterization methods of X-ray Diffraction (XRD), Fourier transform infrared spectroscopy (FTIR), scanning electron microscopy (SEM) and energy dispersive spectroscopy (EDS). These results can provide some theoretical guidance for the application of polymer in CSA-based materials.
2 Experiments
2.1 Raw materials
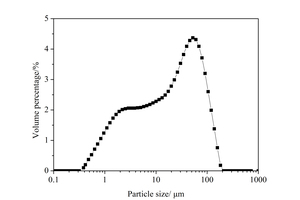
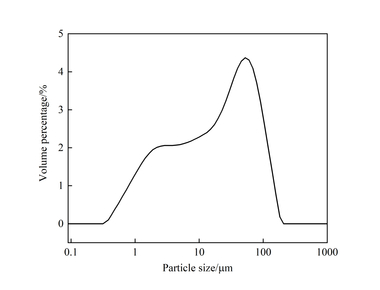
42.5 rapid hardening calcium sulfoaluminate cement with a specific surface area of 440 m2/kg was obtained from Yi’cheng Anda Special Cement Company, and its particle size distribution and chemical composition are shown in Figure 1 and Table 1, respectively. Polycarboxylate superplasticizer (PCE) with a solid content of 40% was used to control the similar fluidity level with the same water/cement ratio. EVA and SBR with a solid content higher than 98% are redispersible latex powders, and their average particle sizes are 150 μm and 85 μm, respectively.
2.2 Sample preparation
The specific mix ratios of the pastes are shown in Table 2. The water-cement ratio was 0.3, and the dosage of EVA and SBR were 2%, 4%, 6% and 8% of the quality of CSA. The fluidity of the paste was controlled to be (200±20) mm with PCE. The specimens were formed in prismatic molds (40×40×160 mm), and then demolded after curing for (24±2) h under the condition of temperature (20±1) °C and relative humidity > 90%. After demolding the specimens were kept in water for 7 d, and then immersed in erosion solution or water and continued to be cured for 28 d, 60 d, 90 d, 120 d and 180 d, respectively. The erosion mode was fully immersed in 5% Na2SO4 solution at (20±1) °C, and the specimens were separated from each other during the immersion to ensure full contact with the erosion solution. The small fragments obtained from the middle part of pastes after the strength test were immersed into anhydrous ethanol for 3 days, and then dried at 40 °C for 12 h. Some small pieces were used for SEM-EDS analysis, and the others were ground into powder by hand, and those particles which could pass the 200-mesh size sieve were used for the XRD and FTIR measurements.
2.3 Test methods
The flexural strengths of CAS pastes were tested according to GB/T 17671-1999 [12] and the erosion resistance coefficient at different ages can be calculated by Equation (1):
⇥ (1)
Where Faq and Fw represent the flexural strength of the sample in erosion solution and water, respectively.
A Bruker D8 Advance X-ray diffractometer (XRD) was used to analyze the phase of the pastes. Nicolet iS50 Fourier transform infrared spectrometer (FTIR) was used to analyze the chemical bonds and functional groups of the hydration products. JSM-6610 scanning electron microscope (SEM) and energy spectrum analysis (EDS) were used to observe the micromorphology and analyze the elemental changes of the eroded samples.
3 Result and discussion
3.1 Flexural Strength
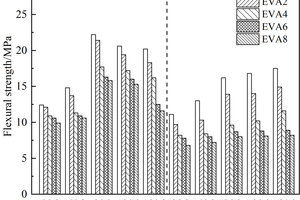
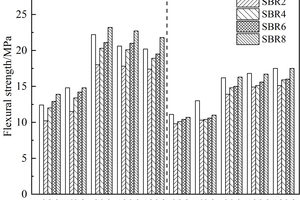
The effects of EVA and SBR on the flexural strength of CSA pastes under sulfate erosion and in water are shown in Figure 2 and Figure 3, respectively.
It can be seen from Figure 2 and Figure 3 that the flexural strength of all samples in sulfate erosion solution showed a trend of first increasing and then decreasing, and the flexural strength of samples under sulfate erosion was higher than that of samples in water. The flexural strength of EVA-doped samples decreased gradually with the increase of EVA dosage, while the flexural strength of SBR-doped samples decreased first and then increased with the increase of SBR dosage, and the flexural strength of SBR-doped samples was higher than that of EVA-doped samples on the whole, mainly because compared with EVA, SBR has smaller particle size and more stress concentration centers, which greatly alleviates the expansion stress of erosion products [13-15]. As a result, the flexural strength of samples mixed with SBR in sulfate solution is higher than that of samples mixed with EVA.
3.2 Sulfate resistance coefficient
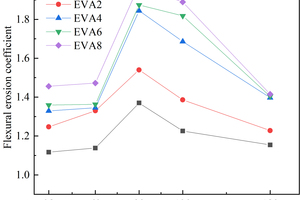
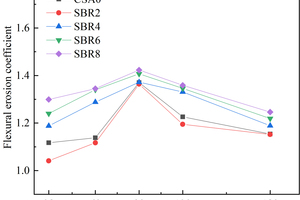
The effects of EVA and SBR on sulfate resistance coefficient of CSA pastes are shown in Figure 4 and Figure 5, respectively.
It can be seen from Figure 4 and Figure 5 that the sulfate resistance coefficient of all samples shows a trend of first increasing and then decreasing. The increasing trend of sulfate resistance coefficient may be due to the reaction of SO42- in the erosion solution with its hydration product to form an expansive substance that fills the pores of CSA paste, thereby increasing its density and flexural strength, thus showing an increasing trend of flexural erosion resistance before 90 d. On the contrary, after 90 d, the swelling materials in the pores of CSA increase, and CSA would crack when the internal stress generated by the swelling materials exceeds the tensile strength of CSA, resulting in the reduction of its flexural strength, which is indirectly reflected in the reduction of the flexural erosion resistance coefficient [16, 17].
With the increase of EVA content, the sulfate resistance coefficient of the samples gradually increased. This may be due to the filling and film formation effects of EVA, which hinder the infiltration of SO42- into the CSA matrix and improve the sulfate resistance of CSA. Different from EVA, the sulfate resistance coefficient of SBR-doped samples showed a trend of first decreasing and then increasing with the increase of the SBR dosage, probably because when the dosage of SBR was 2%, the entraining effect was greater than the film formation effect, which resulted in the reduction of sulfate erosion resistance. When the SBR content is 4%, 6% and 8%, the sulfate resistance coefficient of the sample gradually increases with the increase of the SBR content, which is related to the filling and film forming effect of SBR. SBR particles and their polymerization into films will hinder the erosion of the hydration products of CSA by SO42-, leading to the increase of the erosion resistance coefficient of CSA pastes [18, 19].
At 90 d, the erosion resistance coefficient of each sample reaches the maximum, and the erosion resistance coefficients of CSA0, EVA2, EVA4, EVA6, EVA8, SBR2, SBR4, SBR6 and SBR8 reach 1.370, 1.540, 1.844, 1.874, 1.975, 1.364, 1.372, 1.407 and 1.423, respectively. Usually, the erosion resistance coefficient represents the sulfate resistance of the sample, and the higher the value, the better the sulfate resistance. Therefore, at 90 d, the corrosion resistance of each sample is in the order of EVA8>EVA6>EVA4>EVA2>SBR8>SBR6>SBR4>CSA0>SBR2, which shows that the sulfate resistance of EVA modified CSA pastes is better than that of SBR modified pastes [20, 21].
3.3 XRD analysis
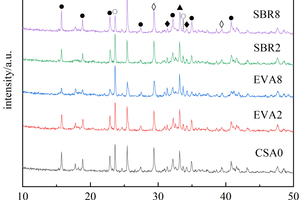
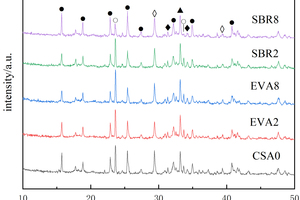
In order to study the effects of EVA and SBR on the hydration of CSA pastes under the condition of sulfate solution erosion and curing in water for 90 d, five samples of CSA0, EVA2, EVA8, SBR2 and SBR8 were selected for XRD analysis, and the results are shown in Figure 6.
According to Figure 6, it can be seen that the main phases of hardened cement pastes under different conditions are ettringite (AFt), dicalcium silicate (β-C2S), anhydrous calcium sulphoaluminate (C4A3S̅), calcium carbonate (CaCO3) and gypsum (CaSO4∙2H2O), and the diffraction peaks in EVA2, EVA8, SBR2 and SBR8 samples are not significantly different from those in CSA0, demonstrating that sulfate erosion and the incorporation of EVA and SBR basically do not change the types of the hydration products of CSA pastes.
Figure 6 also shows that after incorporation of EVA and SBR, the diffraction peak intensity of AFt in the samples under sulfate erosion is higher than that in water curing on the whole, which is related to the reaction of SO42- with hydration products to generate more AFt. Moreover, the order of AFt diffraction peak intensity is SBR8>CSA0>EVA2>SBR2>EVA8 both in sulfate solution and in water. Under the condition of sulfate erosion, the more EVA content, the less AFt production, which is lower than CSA0, probably because EVA particles and their polymerized membranous substances can hinder the combination of SO42- and Ca2+ in the erosion solution to generate more AFt, and the more EVA is added, the more obvious this obstruction is. While for the samples with SBR, the AFt diffraction peak intensity first decreases and then increases with the rise of SBR content, possibly because 2% SBR would introduce some bubbles to form closed pores in the pastes and cut off the connected pores, thus hindering the reaction between SO42- and calcium hydroxide (CH) to generate AFt [22]; As the amount of SBR continues to increase, the hydration products fail to be uniformly dispersed in the polymer, resulting in an increase in the total porosity of the hardened cement paste, and thus more SO42- can react with CH to generate more AFt [23].
3.4 FTIR analysis
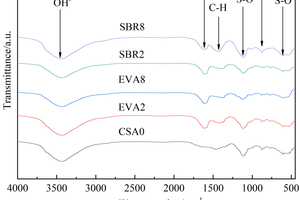
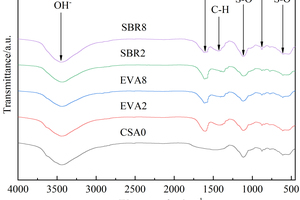
FTIR analysis was conducted on CSA0, EVA2, EVA8, SBR2 and SBR8 samples under the condition of sulfate solution erosion and curing in water for 90 d, and the results are shown in Figure 7.
It can be seen from Figure 7 that under different conditions, C-H bond vibration peaks and benzene ring absorption peaks related to polymer all appear near 1370 cm-1 and 1610 cm-1 in EVA2, EVA8, SBR2 and SBR8 samples. Due to the formation of AFt, an Al-OH peak near 876 cm-1, S-O peaks near 1110 cm-1 and 616 cm-1, and an OH- peak near 3440 cm-1 all appeared in the CSA0, EVA2, EVA8, SBR2 and SBR8 samples [24, 25].
Through the comparison and analysis of Figure 7, it is obvious that the Al-OH vibration peak, S-O vibration peak and OH-vibration peak in CSA pastes gradually decrease with the increase of EVA content, and first decrease and then increase with the increase of SBR content, probably because the incorporation of EVA reduces the content of CH that is easy to cause erosion reaction and the more EVA is added, the less AFt is generated [26]; While 2% SBR is added, some bubbles introduced by SBR would hinder the reaction between SO42- and CH, so that the generation of AFt in SBR2 is less than that in CSA0; When the dosage of SBR increases to 8%, the total porosity of the hardened cement paste increases, and more SO42- in eroded solution reacts with Ca2+, causing the generation of AFt in SBR8 to be higher than that of CAS0.
3.5 SEM and EDS analysis
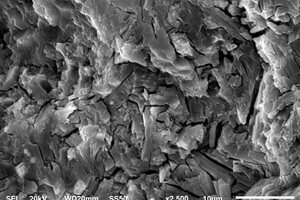
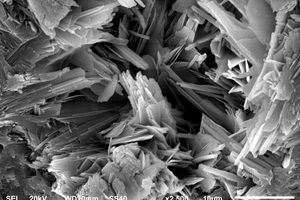
The hydration products and microscopic morphology changes of CSA0, EVA2, EVA8, SBR2 and SBR8 samples were compared and analyzed by SEM-EDS under the condition of sulfate solution erosion and curing in water for 90 d, respectively, and the results are shown in Figure 8 and Figure 9.
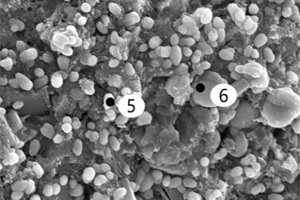
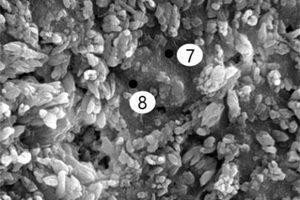
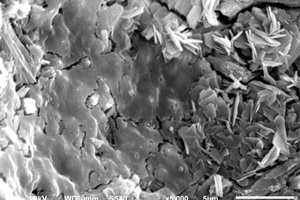
It can be seen from Figure 8 that, after incorporation of EVA and SBR, AFt formed under erosion conditions has more crystals, stronger shape and better internal structural integrity than it does in water. Moreover, there are many internal microcracks in the CSA0 sample, and the sample doped with polymer becomes more compact, because the filling and film formation effects of polymer particles make the internal structure of CSA hardened paste better integrated [27].
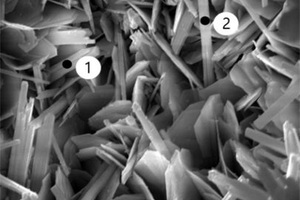
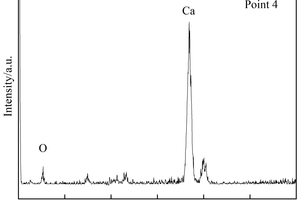
It can be seen from Figure 8 c to Figure 8 f that a large number of needle and rod like substances and some hexagonal plate-like substances are formed in the pores of the EVA2 sample, while the pores in the EVA8 sample are occupied by hexagonal plate-like substances. Energy spectrum analysis of Figure 9 a shows that the needle-rod substance is AFt, and Figure 9 b shows that the hexagonal plate substance is CH. Simultaneously, it can be seen in Figure 8 g that a large number of granular substances fill the gaps between hydration products, which hinder the reaction between SO42- and Ca2+ in erosion solution. Moreover, the more EVA content, the more obvious the obstruction effect, resulting in less AFt production [28].
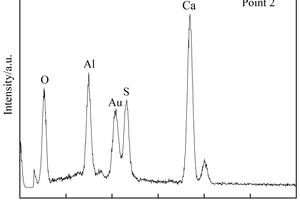
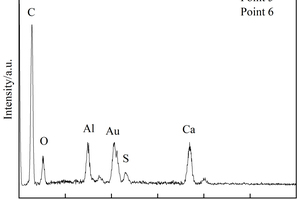
Figure 8 h shows that a large number of polymer particles and membranes formed by polymerization run through the modified CSA system. Figure 8 i ~ Figure 8 l show that AFt in the SBR8 sample is more than that in the SBR2 sample, probably due to the fact that the incorporation of 2% SBR into CSA would also introduce some bubbles, which will hinder the reaction between erosive ions and CH to generate AFt. However, when the SBR content increases to 8%, a large number of SBR particles lead to a larger porosity of CSA paste, which will provide channels for the diffusion of SO42- and then react with Ca2+ to generate more AFt. According to the energy spectrum analysis in Figure 9 c and Figure 9 d, it can be seen that their carbon peaks are relative high, indicating that the granular substances in Figure 8 g are EVA powders, and the membranes in Figure 8 h are SBR polymer films.
4 Conclusion
The flexural strength of EVA-doped samples decreases gradually with the rise dosage of EVA, while the flexural strength of SBR-doped samples decreases first and then increases with the increase dosage of SBR.
The sulfate resistance coefficient of each sample gradually increases with age before 90 d, and then shows a downward trend. At each age, the sulfate resistance coefficient of the samples increases gradually with the increase of EVA content, and decreases first and then increases with the increase of SBR content.
According to the microscopic test, under the condition of erosion of Na2SO4 solution, AFt production of CSA paste at 90 d decreases with the increase of EVA content; When SBR is added with 2%, AFt production decreases, while AFt production increases when SBR content increases to 8%.
5 Acknowledgement
Financial supports from The 14th Five Year plan Hubei Province advantaged characteristic disciplines (groups) project of Wuhan University of Science and Technology (2023D0503) and State Key Laboratory of Silicate Materials for Architectures (Wuhan University of Technology) (SYSJJ2022-20) are gratefully acknowledged.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.