Emission characteristics of NOx and SO2 during the co-combustion of refuse-derived fuels and bituminous coal in a cement precalciner
Aiming at the unstable emission of NOx and SO2 when the cement industry uses refuse-derived fuel (RDF) to partially replace the traditional pulverized coal as fuel for combustion, this paper uses the self-made double-tubular furnace experimental platform to simulate the high temperature and rapid injection of a precalciner. The emission characteristics of CO, NOx, and SO2 from mixed combustion products of bituminous coal and RDF with different qualities were studied at 900 °C. The results show that the release process of CO, NOx, and SO2 over time in the co-burning process of bituminous coal and RDF of different qualities all include volatile stages and carbon fixation stages. In the co-burning process of bituminous coal and RDF of different weights, NOx is mainly formed in the volatile stage when the RDF mixing weight is 0.1 g~0.5 g, the total amount of SO2 in the two stages is the same when the RDF mixing weight is 0.1g, and SO2 is mainly formed in the volatile stage when the RDF mixing weight is 0.2 g~0.5 g. The total amount of NOx increases with the increase of RDF mixing mass. When the RDF mixing mass is 0.3 g, the NOx amount increases less, SO2 emissions are lower, and the sulfur fixation effect is obvious. A good result can be obtained in actual production using RDF with a quality ratio of 60%.
1 Introduction
As a main basic building material, cement plays a key role in the development of the world economy, and the cement industry plays an important role in our industrialization, urbanization, and modernization construction projects. After more than 70 years of development, China’s cement production capacity, varieties, and consumption rank first in the world. By 2021, China’s annual cement output had reached 2.38 billion t, accounting for 54% of the world’s total cement output [1]. However, the rapid development of the cement industry also brings a series of environmental problems....
1 Introduction
As a main basic building material, cement plays a key role in the development of the world economy, and the cement industry plays an important role in our industrialization, urbanization, and modernization construction projects. After more than 70 years of development, China’s cement production capacity, varieties, and consumption rank first in the world. By 2021, China’s annual cement output had reached 2.38 billion t, accounting for 54% of the world’s total cement output [1]. However, the rapid development of the cement industry also brings a series of environmental problems. According to calculations, the cement industry’s NOx emissions account for about 8%~10% of the national emissions, SO2 emissions account for about 3%~4% of the national emissions, and the emission of pollutants is large [2]. The maximum allowable emission concentration of NOx and SO2 in cement kiln and kiln tail waste heat utilization systems is 100 mg/m3 and 50 mg/m3 respectively according to the standard for “Ultra-low Emission of Air Pollutants in the Cement Industry” officially implemented on July 20, 2022. The new standard for NOx, SO2, and other pollutants emission control is stricter, accelerating the green transformation. The mission to find a method to reduce the emission of NOx, SO2, and other pollutantshas become the cement industry’s most urgent task at present.
The emission of NOx and SO2 mainly comes from the combustion of coal in the process of cement clinker firing, so the use of alternative fuel combustion can achieve the purpose of reducing NOx and SO2 emissions during cement production. At present, some cement enterprises have carried out relevant technology research and development applications, and the feasibility of alternative fuel combustion is verified. For example, Conch Group and Kawasaki Company of Japan jointly developed the technology of combining the new-type dry kiln and gasifier in the cement industry to treat municipal solid waste, the gasifier being used for waste pretreatment, and the combustible gas generated being used as an alternative fuel for cement production [3]. Based on Holcim Group’s experience in waste treatment technology and combined with the characteristics of domestic household waste, Huaxin Company and Holcim Group jointly developed the treatment technology of collaborative disposal of solid waste in cement kilns. In the stage of waste pretreatment, the waste is dried and dehydrated, and then the fuel it contains is separated and used as an alternative fuel for cement production [4]. Sinoma International has independently developed a complete system for the collaborative disposal of MSW by using a new dry cement kiln, which directly separates the original ecological MSW into combustibles and feeds it into the precalciner for use as fuel [5]. However, due to unclear emission characteristics of NOx and SO2 in the co-combustion process of RDF and bituminous coal, enterprises generally adopt rough delivery, and there is a situation of “one kiln, one working condition, one kiln, one scheme”, resulting in unstable emission of pollutants.
The use of alternative fuels in cement kilns is the strategic direction of the development of the Chinese cement industry, which conforms to the energy strategy of optimizing the energy structure. The use of municipal waste to prepare alternative fuels for the combustion of cement kilns can generate heat from the combustible components of waste and reduce the impact of NOx, SO2, and other toxic and harmful pollutants on the environment. Refuse-derived fuel (RDF), one of the products of municipal solid waste (MSW), has been used effectively as an energy source, especially in the cement industry [6]. RDF are made by crushing, sorting, drying, adding agents, compression molding, and other treatments of combustible wastes. They are characterized by stable combustion, easy storage, and low secondary pollution [7-11]. In theory, RDF is a good alternative fuel to coal because it contains high volatilities and can promote ignition [12-15]. Some studies have been conducted on RDF and coal. For example, Botakoz Suleimenova [16] mixed RDF with high-ash bituminous coal and co-fired it in a laboratory-scale bubbling-fluidized bed reactor (BFB) at a bed temperature of 850 °C. Adam Smolińskia [17] used RDF and bituminous coal oxygen/water vapor co-gasification to produce hydrogen-rich gas, he [18] also proposed co-combustion of low-grade coal and municipal solid waste. Izabella Maj [19] studied the water wall corrosion products of co-fired RDF in CFB multi-fuel boilers. Agata Mlonka-Mędrala [20] analyzed in detail a dry adsorbent’s influence on the composition, morphology, and properties of fly ash in circulating fluidized bed scrubber and bag filter. J. F. Lu [21] studied the co-combustion of refuse-derived fuel (RDF) and coal in a circulating fluidized bed combustor (CFBC). Xiaolin Wei [22] used an internal circulating fluidized bed (ICFB) to study the behavior of chlorine and sulfur in the co-firing process of RDF and coal. Kechang Xie [23] developed a novel internal circulating fluidized bed (ICFB) for mixed combustion of coal and refuse-derived fuel (RDF). Most of their experimental studies were conducted in fluidized beds, which are different from the cement precalciner environment. To stabilize and reduce NOx and SO2 emissions in the cement industry, it is necessary to have a thorough understanding of the NOx and SO2 generation process in the precalciner environment. Therefore, this study takes standard coal with properties similar to those of bituminous coal used in cement plants and the actual used RDF as the research object, uses the double-tubular furnace experiment platform to simulate the oxygen concentration and high-temperature environment in the precalciner, studies its combustion characteristics, and seeks a suitable alternative fuel to stabilize the proportion and reduce NOx and SO2 emissions. The research results of this paper can provide a reference for the actual cement enterprises that adopt the alternative fuel technology to save energy and reduce emissions, and provide a theoretical basis for their industrial treatment and new energy technology.
2 Experiment
2.1 Experimental materials
The RDF used in this experiment is an alternative fuel used by a cement company in Hubei Province, and it comes from municipal household waste in Wuhan. As a natural mineral, coal generally has certain fluctuations of trace components. To ensure the stability of C, N, and other components in the coal sample in the process of this study and avoid the contingency of experimental results, standard coal is selected as the experimental coal in this experiment. The proximate analysis and ultimate analysis of RDF and coal are shown in Table 1.
2.2 Experimental facility
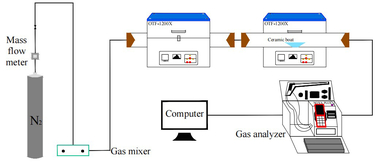
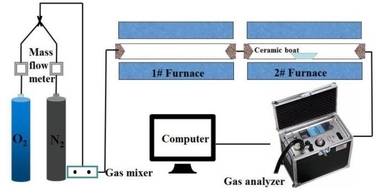
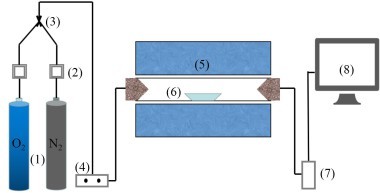
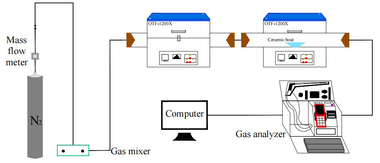
This experiment designed and established the constant temperature experiment platform of the double tubular furnace to simulate the high temperature and hot gas flow environment in the precalciner. The double tubular furnace system (see Figure 1) includes a gas configuration system, a high-temperature tubular furnace, and a flue gas analyzer. The model of the two high-temperature tubular furnaces is OTF-1200X, and the heating element is silicon carbon tube. The temperature can be adjusted from room temperature to 1200 °C. The furnace temperature is digitally displayed and controlled by the automatic regulator. The gas required for combustion is a mixture of N2 and O2. The nova plus flue gas analyzer was used to analyze the flue gas composition after fuel combustion. The flue gas analyzer was connected to the computer through the RS232 interface for online data collection.
2.3 Experimental methods
Connect the experimental device as shown in Figure 1, turn on the flue gas analyzer, and preheat it for 30-40 min. After the flue gas analyzer starts normally and enters the monitoring interface, the whole reaction system ventilation is checked for cold air tightness. The tubular furnace is set to heat up to the set temperature of 900 °C at the rate of 10 °C/min and keep warm. Weigh 0.5 g bituminous coal and RDF samples of different qualities, mix evenly, and lay them on a 60 mm × 30 mm × 15 mm corundum porcelain boat. Open the gas cylinder, adjust the gas flowmeter, and set O2 concentration 18%, and N2 concentration 82%, the total gas mass flow rate being 1000 ml/min, and ventilate for 10-20 min to make the gas in the environment full and stable. When the gas indicator on the flue gas analyzer is stable, the porcelain boat loaded with samples is quickly pushed to the middle position of the tubular furnace, and continuous measurement is started until the detection results of CO, NOx and SO2 gas are all reduced to 0 mg/l, and the recording is stopped. O2 and CO2 were measured by percentage, while CO, NOx, and SO2 were measured by mg/l and set to be recorded every 1 s. The gas release curve used time as the horizontal coordinate.
3 Results and discussion
3.1 CO formation in mixed combustion of bituminous coal and RDF
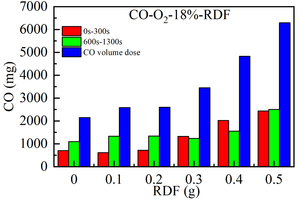
Figure 2 shows the release curve of CO over time when 0.5 g bituminous coal is mixed and burned at 900 °C, O2 concentration is 18% and RDF of different qualities is mixed. Figure 3 shows the cumulative amount of CO gas released by integrating the CO release curve over time when bituminous coal is mixed with RDF combustion of different qualities. Table 2 shows the peak concentration and corresponding formation time of CO when bituminous coal is burned with different RDF mixing weights.
Combined with Figure 2 and Table 2, it can be seen that when bituminous coal is mixed with RDF combustion of different qualities, the release curve of CO over time shows two sets of peaks: a “sharp peak” with rapid first increase and then decrease, and a gentle “steamed bun peak”. “Sharp peak” width is 0 s-300 s, and is caused by rapid combustion of volatile, which can be called volatile CO peak; The starting point of “steamed bun-shaped peak” is t = 600 s and ends between t = 1200 s – 1300 s, corresponding to the combustion of fixed carbon, which can be called fixed carbon type CO peak.
From the perspective of peak size and formation time of CO (combined with Figure 2, Figure 3 and Table 2), with the increase of RDF mixing mass, the peak concentration of volatile CO decreases first and then increases, in order (12 mg/l, 4 mg/l, 7 mg/l, 16 mg/l, 23 mg/l, 25 mg/l). The formation time of peak concentration of volatile CO increases continuously (27 s, 28 s, 29 s, 32 s, 50 s, and 60 s). When the ratio of RDF is 0%, the peak formation rate of volatile CO is the fastest (26 s), and the peak concentration is 12 mg/l, which is between 0.2 g and 0.3 g of RDF mixing mass. As the mixing weight of RDF increases from 0.1 g to 0.5 g, the formation time and peak concentration of volatile CO increase continuously. This is because RDF contains a large number of volatile components (71.83%). With the increase of the mixing weight of RDF, the volatile content in the total sample increases significantly, and the time required for combustion and the concentration of volatile CO produced also increase correspondingly. Combined with the combustion curve of single bituminous coal, it can be seen that the addition of more RDF weight (0.3 g~0.5 g) is conducive to the formation of the CO rich reduction region. Due to the small amount of fixed carbon in RDF (9.11%), the peak value of fixed carbon CO does not change significantly. When the mixing mass of RDF is between 0 g and 0.5 g, the release curve of fixed carbon CO tends to be flat and the peak concentration is very low, staying between 2 mg/l and 4 mg/l.
From the perspective of the total amount of CO (combined with Figure 2 and Figure 3), with the increasing RDF mixing quality, the total amount of CO released by the mixed combustion of bituminous coal and RDF is 0 g < 0.1 g, 0.2 g < 0.3 g < 0.4 g < 0.5 g. When the mixing mass of RDF ranges from 0 g to 0.2 g, the release peak width of CO is very narrow and the peak value is low, and the increase in the total release of CO is small. The total amount of CO produced in the combustion stage of fixed carbon is significantly more than that in the volatile combustion stage, and fixed carbon combustion dominates. When the mixing mass of RDF is 0.3 g to 0.5 g, the release peak width of CO is wider and the peak value is higher, and the peak width is 4-6 times that when the mixing mass is 0 g to 0.2 g, and the total amount of CO released increases greatly. When the mixing mass of RDF is 0.3 g and 0.5 g, the total amount of CO produced in the volatile combustion stage is equivalent to that in the fixed carbon combustion stage. When the mixing weight of RDF is 0.4 g, the total amount of CO produced in the volatile combustion stage of CO is significantly more than that in the fixed carbon combustion stage, and the volatile combustion dominates. Compared with single bituminous coal combustion, it can be seen that when the mass of mixed RDF is 0.1 g and 0.2 g, it has little influence on the total amount of CO produced by bituminous coal combustion, and the combustion is more complete. When the mixing weight of RDF increases from 0.3 g to 0.4 g and 0.5 g, the total amount of CO produced by incomplete combustion increases gradually due to the coverage of combustible components by the high ash content of RDF (17.93%), and the instantaneous precipitation of high volatile content (71.83%) to form a “fuel-rich oxygen-poor zone”.
In summary, the combustion process of RDF mixed with bituminous coal consists of two stages: volatile release, combustion, and combustion of fixed carbon. There are two sets of peak values in the release curve of CO over time during the mixed firing of bituminous coal and RDF. The peak value of volatile CO first decreases and then increases with the increase of mixing amount, and the formation time gradually increases with the increase of mixing amount. The mixing amount does not affect the peak value and formation time of fixed carbon CO. When the mixing amount of RDF is between 0 g and 0.2 g, the fixed carbon type CO dominates. When the mixing amount of RDF is 0.3 g and 0.5 g, the total amount of CO in the two stages is similar. When the mixing weight of RDF is 0.4 g, the volatile type CO dominates. When the mixing weight of RDF exceeds 0.3 g (mass ratio 60%), the total amount of CO increases greatly.
3.2 NOx formation in mixed combustion of bituminous coal and RDF
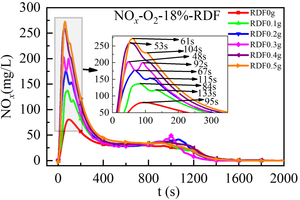
Figure 4 shows the NOx release curve over time when 0.5 g bituminous coal is mixed with RDF of different qualities for combustion at 900 °C, O2 concentration is 18% and the temperature is 900 °C. Figure 5 shows the NOx emission cumulative amount obtained by integrating the NOx release curve over time when bituminous coal is mixed with RDF combustion of different qualities. Table 3 shows the peak NOx concentration and corresponding formation time when bituminous coal is burned under different RDF mixing weights.
Combined with Figure 4 and Table 3, the NOx release rule is analyzed when bituminous coal is mixed with RDF of different qualities at the furnace temperature of 900 °C. It can be seen that the NOx release rule is the same when the bituminous coal is mixed with RDF of different qualities, and there are two sets of peaks: 0 s-300 s forms a “sharp peak” that increases first and then declines, and 600 s-1300 s forms a gentle “steamed bun shaped peak”. The two formation stages of NOx correspond to CO and can be defined as volatile type NOx peak and fixed carbon type NOx peak.
From the perspective of NOx peak size and formation time (combined with Figure 4, Figure 5, and Table 3) at the volatile combustion stage of 0 s-300 s, a “sharp peak” is formed when the mixing mass of RDF is 0 g and 0.4 g, and two consecutive “sharp peaks” are formed when the mixing mass of RDF is 0.1 g, 0.2 g, 0.3 g, and 0.5 g. The first peak is caused by the rapid release of volatile elements from the fuel, and the second peak may be caused by the release of nitrogen-containing elements in some RDF samples due to the non-uniformity of RDF components. Compared with single bituminous coal combustion, the generation rate of volatile NOx increases after being mixed with RDF, and the peak concentration of volatile NOx increases gradually with the increase of the mixed mass of RDF. The generation rate of fixed carbon NOx is similar to the peak value during combustion after mixing RDF, and the amount of RDF mixing has little influence on this stage. When the mixing mass of RDF is 0.3 g, the NOx formation rate is the fastest, and the maximum concentration value is formed at t = 48 s. Although the NOx release rate is fast during high-temperature combustion, the high concentration of CO inhibits the generation of NOx and more generated NOx is reduced. Therefore, the peak concentration of volatile NOx of bituminous coal is low when the mixed RDF mass is 0.3 g.
From the perspective of the total amount of NOx (combined with Figure 4 and Figure 5), the total amount of NOx released by the mixed combustion of bituminous coal and RDF increases with the increasing mixing quality of RDF. When bituminous coal is burned alone, the total amount of NOx produced in the fixed carbon combustion stage is equivalent to that in the volatile combustion stage. When the mixing mass of RDF is 0.1 g~0.5 g, the total amount of NOx produced in the volatile combustion stage is significantly more than that in the fixed carbon combustion stage, so the volatile combustion dominates the whole combustion process. When the mixing mass of RDF is 0 g, 0.1 g, and 0.2 g, the total amount of NOx released increases greatly. When the mixing mass of RDF is 0.3 g, the total amount of NOx released is equivalent to that of RDF when the mixing mass of RDF is 0.2 g, but the total amount of NOx released is much different from that of RDF when the mixing mass of RDF is 0.4 g and 0.5 g. When the mixed mass of RDF is 0.1 g, the amount of volatile type NOx is the lowest, because the added mass of RDF is the least, and the released CO makes it difficult for the nitrogen-containing material to convert to the direction of NOx. When the mixing mass of RDF is 0.5 g, the amount of volatile type NOx is the largest, because the added mass of RDF is the largest, and RDF contains a large number of volatile components (71.83%) that can be burned to produce NOx.
In summary, the NOx release curves with time in the mixed firing process of bituminous coal and RDF show two sets of peaks, among which the peak value of volatile NOx gradually increases with the increase of mixing amount, the formation time first decreases and then extends with the increase of mixing amount, and the peak value and formation time of fixed carbon NOx are not affected by the mixing amount. When the mixing amount of RDF is 0 g, the total amount of NOx in the two stages is the same, and when the mixing amount of RDF is 0.1 g to 0.5 g, volatile type NOx dominates. When the mixing mass of RDF is 0.3 g (mass ratio 60%), the peak value of volatile NOx appears the earliest (48 s), the reaction rate is the fastest at this time, and the total amount of NOx increases less, which is the appropriate mixing ratio of RDF.
3.3 SO2 formation in mixed combustion of
bituminous coal and RDF
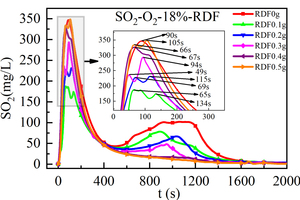
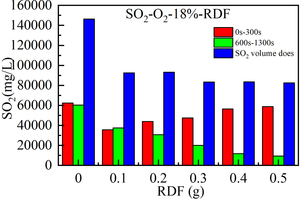
Figure 6 shows the release curve of SO2 over time when 0.5 g bituminous coal is burned at 900 °C, O2 concentration is 18% and RDF of different qualities is mixed. Figure 7 shows the cumulative amount of SO2 gas release obtained by integrating SO2 release curve over time when bituminous coal is mixed with RDF combustion of different qualities. Table 4 shows the peak SO2 concentration and corresponding formation time when bituminous coal is burned with different RDF mixing weights.
Combined with Figure 6 and Table 4, it can be seen that when bituminous coal is mixed with RDF combustion of different qualities, the release curve of SO2 over time also has two sets of peaks: 0~300 s forms a “sharp peak” that increases first and then decreases; 600 s-1300 s forms a gentle “steamed bread peak”. Similar to the combustion stage of CO and NOx, the release process of SO2 is also divided into two stages: volatile combustion (0 s-300 s) and fixed carbon combustion (600 s-1300 s).
From the perspective of the size and formation time of the peak SO2 (combined with Figure 6, Figure 7, and Table 4), compared with the single bituminous coal combustion, the peak concentration of volatile SO2 and fixed carbon SO2 decreased after mixing RDF, indicating that RDF has a sulfur fixation effect. When the mixing mass of RDF increases from 0.1 g to 0.5 g, the peak value of volatile SO2 concentration increases continuously, and there are two consecutive peaks in part of the curve. The first peak value is the formation of volatile sulfur combustion, and the second peak value is the formation of partial sulfur minerals decomposition. In the volatile combustion stage (0 s-300 s), the formation time of SO2 concentration peaks is the fastest (49 s, 94 s) when the mixing mass of RDF is 0.3 g. At the stage of fixed carbon combustion (600 s-1300 s), the peak concentration of SO2 decreases with the increasing mixing mass of RDF, which may be attributed to the fact that the metal oxides contained in RDF ash react with SO2 to form sulfate, leading to a decrease in the concentration.
From the perspective of the total amount of SO2 (combined with Figure 6 and Figure 7), with the increasing RDF mixing quality, the total amount of SO2 released by the mixed combustion of bituminous coal and RDF is 0 g > 0.1 g, 0.2 g > 0.3 g, 0.4 g, 0.5 g, indicating that the addition of RDF has a good sulfur fixation effect. When the mixing mass of RDF is 0 g and 0.1 g, the total amount of SO2 produced in the volatile combustion stage is equivalent to that produced in the fixed carbon combustion stage. When the mixing weight of RDF is 0.2 g~0.5 g, the total amount of SO2 produced in the volatile combustion stage is more than that produced in the fixed carbon combustion stage, so the volatile combustion stage dominates the whole combustion process. After mixed combustion with RDF, the total amount of SO2 produced decreases significantly compared with that of bituminous coal alone, which may be due to the good adsorption and removal of acid gas (SO2) by a large number of calcium-based substances contained in RDF, leading to a decrease in the amount of SO2 produced. By comparing the mixed RDF with a mass of 0.1 g to 0.5 g, it can be seen that when the mixed RDF has a mass of 0.1 g and 0.2 g, the total amount of SO2 released is similar and high. When the mixing mass of RDF is 0.3 g, 0.4 g, and 0.5 g, the total release of SO2 decreases, indicating that the sulfur fixation effect is better when the mixing mass of RDF is larger (0.3 g~0.5 g) [15].
In summary, the release curves of SO2 with time in the mixed firing process of bituminous coal and RDF show two sets of peaks. The peak value of volatile SO2 first decreases and then increases with the increase of mixing amount, the formation time first decreases and then extends with the increase of mixing amount, and the peak value of fixed carbon SO2 gradually decreases with the increase of mixing amount, and the formation time is similar. When the mixing amount is 0 g and 0.1 g, the total amount of SO2 in the two stages is similar. When the mixing amount is 0.2 g~0.5 g, the volatile SO2 is dominant. More than 0.3 g (mass ratio 60%) of RDF has a better sulfur fixation effect.
4 Conclusions
The release process of CO, NOx and SO2 over time shows the same rule in the co-burning process of bituminous coal and RDF of different qualities, and the release curve over time shows two sets of peaks, including the volatile stage and the fixed carbon stage.
In the co-burning process of bituminous coal and RDF of different weights, the total amount of NOx in the two stages is the same when the mixing weight of RDF is 0 g, and the NOx is mainly formed in the volatile stage when the mixing weight of RDF is 0.1 g~0.5 g. When the mixing weight of RDF is 0 g and 0.1 g, the total amount of SO2 in the two stages is equivalent. When the mixing weight of RDF is 0.2~0.5 g, SO2 is mainly formed in the volatile stage. The research and application of denitrification and desulphurization technology of bituminous coal and RDF mixed firing should focus on the formation stage of volatile NOx and SO2.
The total amount of NOx increases with the increase of RDF mixing mass. When the RDF mixing mass is 0.3 g, the NOx amount increases less, SO2 emissions are lower, and the sulfur fixation effect is obvious. In actual production, good results can be achieved when the mixing quality ratio of RDF is 60%.
Acknowledgments
This work was financially supported by the scientific research plan guiding project of the education department of Hubei Province, China (B2021011) and 2022 innovation and entrepreneurship training program for university students of Hubei Province (S202210488088).

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.