Numerical simulation of oxygen-enriched combustion in a rotary kiln in the primary air and the secondary air respectively
Aiming at a real-size rotary kiln, numerical simulations were carried out for air combustion, primary air oxygen enrichment, and secondary air oxygen enrichment conditions, respectively, in order to explore the influence of oxygen-enrichment methods on heat transfer and NOx generation in the rotary kiln. The results show that by using oxygen-enriched combustion
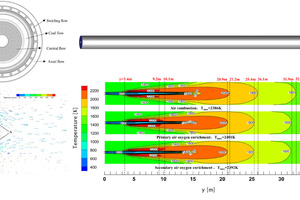
technology, the shape of the high temperature zone generated by the combustion is almost unchanged, in the shape of a willow leaf, and as the combustion rate of volatiles and char increases, the overall temperature of the flame increases, and thus the amount of radiation received by the wall surface of the rotary kiln also increases. Comparing the two oxygen enrichment methods, it can be found that the oxygen enrichment in the primary air results in the most obvious increase in average temperature, and the total radiation received by the wall surface in the burning zone is increased by about 6.76%, but the NOx generation is also increased significantly, with the NOx at the outlet increasing from 1099 ppm to 1303 ppm.
1 Introduction
China is a major cement producer and consumer. According to statistics, China produced 2.4 billion t of cement in 2020 [1]. The cement industry is also the main coal consumption and CO2 emission industry, it is the third top CO2 emitter industry in the world, responsible for 5% of global CO2 emissions [2], which brings great challenges with regard to the sustainable development of the cement industry. Therefore, improving the efficiency of pulverized coal combustion and reducing coal consumption and CO2 emissions have become urgent problems for the cement industry.
As an important...
1 Introduction
China is a major cement producer and consumer. According to statistics, China produced 2.4 billion t of cement in 2020 [1]. The cement industry is also the main coal consumption and CO2 emission industry, it is the third top CO2 emitter industry in the world, responsible for 5% of global CO2 emissions [2], which brings great challenges with regard to the sustainable development of the cement industry. Therefore, improving the efficiency of pulverized coal combustion and reducing coal consumption and CO2 emissions have become urgent problems for the cement industry.
As an important thermal equipment in the cement production process, the rotary kiln has multiple functions, such as fuel combustion, clinker calcination, and material transportation. In the process of pulverized coal combustion and clinker calcination, a large amount of N2 in the air is heated, and part of the heat is dissipated through the high-temperature flue gas, resulting in heat loss. Studies have shown that by using oxygen-enriched combustion technology to increase the O2 content in the combustion air, the flame temperature and blackness can be increased, the flue gas radiation can be enhanced, and the exhaust gas volume and heat loss can be reduced [3-6].
With the development of computer technology, CFD numerical simulation technology has become an important means of flow field analysis in the rotary kiln [7-10]. A lot of research work has been carried out on the oxy-fuel combustion of rotary kiln. Li Song [11] found that the addition of an oxygen enriched channel in the rotary kiln will not have a significant impact on the velocity field, and can promote the combustion of pulverized coal in the rotary kiln, which verified the feasibility of oxygen enriched combustion. Mingyue, W. [12] and Bin, L. [13] studied the effect of oxygen enrichment in primary air on the combustion characteristics of pulverized coal in a rotary kiln, and found that as the oxygen concentration increases, the maximum temperature and combustion efficiency increase, but the temperature uniformity declines.
Many studies on oxygen-enriched combustion only focus on the effects of primary air oxygen concentration on flame shape, temperature distribution, NOx and other parameters, but have not studied and compared the effects of secondary air oxygen enrichment on rotary kilns under the same oxygen content. In view of this, numerical simulations were carried out aimed at a traditional air combustion rotary kiln. In the case of the same oxygen flow rate, the primary air and the secondary air were enriched with oxygen respectively, and the parameters such as flame shape, temperature distribution, NOx and other parameters in the rotary kiln were compared. The research results have important theoretical reference significance for the implementation of the oxygen-enriched combustion process of the rotary kiln.
2 Geometric models and meshes
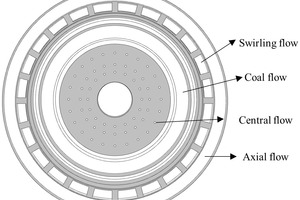
The structure of the rotary kiln is shown in Figure 1, and its size is Φ 4.5 m × 74 m. A four-channel burner is used in this rotary kiln, in which there are central air, coal air, swirling air and axial air from inside to outside, as shown in Figure 2.
The grid division of the rotary kiln shell is shown in Figure 3. Considering that there is usually strong turbulence at the end of the burner, the flow field will vary greatly, so the mesh is refined here.
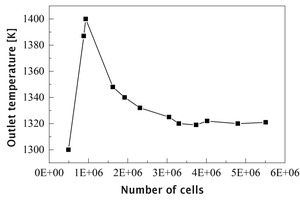
In the simulation process, in order to ensure the accuracy of the calculation it is necessary to determine whether the simulation results change with the change of the number of grids, that is, to verify the grid independence. Figure 4 shows the curve of the average temperature of flue gas at the outlet as a function of the total amount of rotary kiln grids. It can be found that when the number of cells is greater than 3.3 million, the average outlet temperature is basically unchanged, and the fluctuation is within the allowable error range, so this number of grids is used for calculation
3 Numerical model
3.1 Gas phase
For the gas phase, the RNG k-ε model is used to simulate the turbulent flow. When there is a swirling flow, the RNG k-ε turbulence model has higher prediction accuracy and greater convergence stability [14]. The model is defined as:
⇥
⇥ (1)
⇥
⇥(2)
⇥
⇥(3)
In these equations, Gk represents the turbulence kinetic energy generation due to the mean velocity gradients. Gb is the turbulence kinetic energy generation due to the buoyancy. YM represents the contribution of the fluctuating dilatation in the compressible turbulence to the overall dissipation rate. C2 and C1ε are constants. σk and σε are the turbulent Prandtl numbers for k and ε, respectively. Sk and Sε are user-defined source terms.
3.2 Particle phase
For the particle phase (pulverized coal), the discrete phase model and discrete random walk model are used to calculate the trajectory of the particle phase. It is assumed that the particles are spherical and the diameter distribution conforms to the Rosin-Rammler distribution, without considering the interaction between pulverized coal particles.
For the pulverized coal combustion process, the species transport model combined with the Finite-Rate/Eddy-Dissipation model was used. The combustion process of pulverized coal is divided into two parts. Volatile components are first released and burned quickly, during which the char begins to burn. The single-kinetic-rate devolatilization model was used for the volatile devolatilization, which assumes that the rate of devolatilization is first-order and depends on the amount of volatiles remaining in the particle [15].
For char combustion, the kinetic/diffusion surface reaction rate model was used, which assumed that the surface reaction rate was determined either by kinetics or by diffusion rate [16-17]. The P-1 radiation model was used to calculate the radiation heat transfer in the rotary kiln.
3.3 NOx model
The pollutant model was used to calculate the generation of NOx in the rotary kiln. Considering the generation of thermal NOx and fuel NOx in the rotary kiln, and NOx reduction by reburning, the model is set as follows:
1. Thermal NOx
The formation of thermal NOx is determined by a set of highly temperature-dependent chemical reactions known as the extended Zeldovich mechanism. The principal reactions governing the formation of thermal NOx from molecular nitrogen are as follows:
⇥(4)
⇥(5)
Through the above reaction, it is found that in order to simulate the reaction process of thermal NOx, in addition to knowing the content of stable components (O2, N2), it is also necessary to know the concentration of [N] and [O]. Assuming that the two atomic concentrations are partial-equilibrium.
2. Fuel NOx
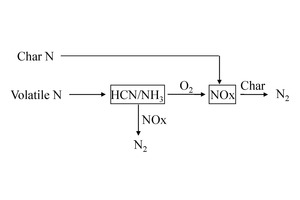
For coal, fuel nitrogen is assumed to be distributed in volatiles and char, the reaction principle is shown in Figure 5. Volatile N reacts at a certain temperature to form HCN and NH3, one part reacting with oxygen to form NOx, and the other part reacting with the produced NOx to form N2. For the remaining char N, it is assumed that it directly reacts with oxygen to generate NOx, without intermediate products.
3. NOx reduction by reburning
Pulverized coal releases a large amount of volatile matter in the early stage of combustion, and the volatile matter contains a large amount of hydrocarbons, the main components of which are coal tar and CH4. When the reaction temperature range is 1600K-2100K, the reaction equation is as follows, where the reaction products are O, OH, and H2O.
⇥(6)
4 Boundary conditions and numerical scheme
The real data under the actual working condition of the rotary kiln was used as the boundary condition. Inlet velocity for primary air inlet and secondary air inlet with the detailed boundary conditions of each inlet under air combustion shown in Table 1. Pressure-outlet for outlet. The wall temperature of the rotary kiln is set to 1300 K. It is ensured that the primary air velocity and the total O2 at the inlet remain unchanged under the three conditions of air combustion, primary air and secondary air oxygen enrichment. The specific boundary conditions are shown in Table 2, and the proximate and ultimate analysis of coal are shown in Table 3.
The control equations of the fluid phase were discretized by the finite volume method. The difference equations were solved using a second-order upwind difference scheme. The pressure and velocity were coupled using Coupled algorithm. The Standard scheme was adopted for pressure discretization. The equations were numerically solved by the tridiagonal matrix algorithm (TDMA) method. The process was repeated until convergence was achieved with the convergence criterion being less than 10-6 for the energy and P-1 term, and less than 10-3 for the remaining residuals.
5 Result and discussion
5.1 Result verification
The comparison between the simulated data and the measured data under the air combustion condition is shown in Table 4. The measured temperature data is from the central control room of the cement plant, and the outlet gas composition data is the actual test data of the flue gas analyzer. It can be seen that the error between the simulated value and the actual measured data is very small, which is within the allowable error range of the project. Taking into account the complexity of the clinker calcination, the pulverized coal combustion chemical reaction, and the error of the numerical simulation calculation itself, it shows that the simulation results are reasonable and can reflect the actual working conditions in the rotary kiln.
5.2 Gas flow fields
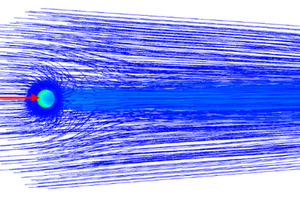
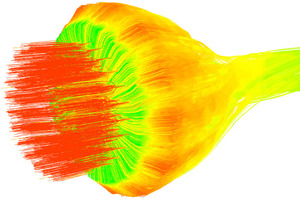
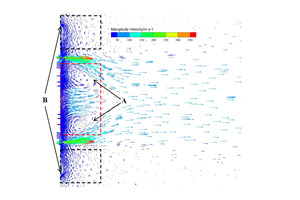
The overall gas streamlines, the secondary air streamlines, and the central air streamlines are shown in Figures 6, 7, and 8. It can be seen from Figures 6 and 7 that the secondary air near the burner is entrained by the primary air from the burner, From Figure 8 it can be observed that due to the influence of the swirling air, the central air is “twisted” into a slender stream. Figure 9 shows the velocity vector on the central section of the burner. Combining Figure 2 and Figures 6-9, it can be seen that there are two obvious recirculation zones (“A” and “B”) in the burner head area. The “A” area is formed due to the inward rotation of a part of the central air, which plays the role of gathering the flames. The “B” area is the external recirculation zone, which is formed by the high-speed axial air of the outermost layer of the burner entraining high-temperature secondary air. The formation of the external recirculation zone is conducive to the mixing of pulverized coal and high-temperature secondary air, thereby accelerating the ignition and combustion rate of pulverized coal.
5.3 Coal combustion mechanism
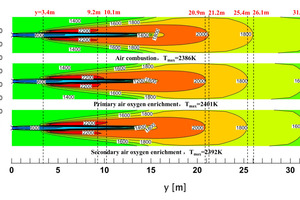
In the rotary kiln, the combustion of pulverized coal provides heat for the calcination of clinker; a reasonable flame shape and thermal regulation are the basis for stable production. Figure 10 shows the isosurface map of CO which indicates a willow leaf-like flame. Figure 11 shows the temperature contour along the axial section of the rotary kiln under three working conditions (air combustion, primary air oxygen enrichment and secondary air oxygen enrichment). From the perspective of the high temperature zone, the high temperature zones corresponding to the three working conditions are all “willow leaf-like” with no obvious change in overall shape. The length of the black fire head is almost the same, both are about 3 m, which is caused by the same primary air velocity under the three working conditions; Compared with the oxygen-enriched conditions, the “flame” length under the air combustion conditions is slightly longer, and the “flame” lengths under the two oxygen-enriched conditions are almost the same. This is because the secondary air velocity in the air combustion condition (8.26 m/s) is slightly higher than that in the two oxygen-enriched conditions, and the secondary air velocity in the two oxygen-enriched conditions (7.99 m/s) is the same.
Compared with the air combustion conditions, the maximum temperature in the kiln corresponding to the two oxygen-enriched conditions is increased, in which the maximum temperature of oxygen enrichment in primary air and secondary air is increased by 15 °C and 6 °C respectively. In addition, the coverage area of the high temperature zone (2200 K) in the initial stage of pulverized coal combustion became larger, and the coverage length increased by about 1 m, which provided favorable conditions for calcining clinker in the burning zone.
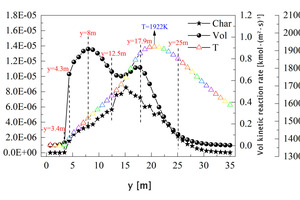
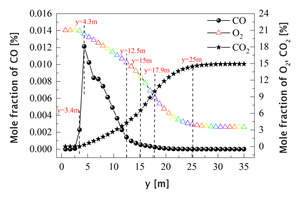
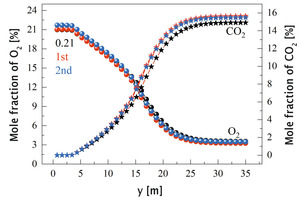
In order to further explore the combustion mechanism of pulverized coal, the average volatile kinetic reaction rate curve, char burnout rate curve and average temperature curve from 0 m to 35 m along the length of the kiln under the air combustion condition are drawn, as shown in Figure 12. Figure 13 shows the average mole fraction of CO, O2 and CO2 along y direction. The combustion process of pulverized coal is divided into two parts, namely volatile combustion and char combustion. Combining Figure 12 and Figure 13, it can be seen that at y=3.4 m, the volatile begins to release and burn rapidly, generating a large amount of CO (Figure 13), and the char begins to burn slowly. At y=4.3 m, the combustion rate of volatile increases suddenly and the concentration of CO reaches its peak at this position. In the range of 4.3 m<y<12.5 m, the volatile is burned intensively. At y=8 m, the volatile combustion rate reaches its peak. At this stage, the combustion of pulverized coal is fuel-rich combustion, so a large amount of CO is produced. With the combustion of volatile matter, the burnout rate of carbon at this stage begins to increase further. In the range of 12.5 m<y<17.9 m, as the volatile content gradually decreases, char combustion begins to dominate, and its burnout rate increases rapidly. The main combustion product is CO2, and some CO is also generated. In the range of 17.9 m<y<25 m, the reaction enters the late stage, the kinetic reaction rate of volatile and the burnout rate of char both decrease rapidly, the temperature begins to fall after reaching its peak, the CO concentration gradually approaches 0, while the CO2 concentration tends to stabilize. When y>25 m, only a small amount of unburned pulverized coal continues to burn.
When the oxygen concentration in the combustion-supporting gas increases, the combustion process of pulverized coal will be affected. Figures 14 and 15 show the average volatile kinetic reaction rate and the char burnout rate along y direction under the conditions of air combustion, primary air oxygen enrichment and secondary air oxygen enrichment, respectively.
As shown in Figure 14, the volatile combustion processes under the three working conditions are similar. After the volatile is released, it begins to burn rapidly at y=4.3 m, and reaches the maximum rate at y=8 m. Then, due to the gradual decrease of volatile matter and the influence of the competitive combustion of char, the burning rate of volatile matter slows down and peaks again at y=17.9 m (air combustion condition) and at y=17 m (oxygen-enriched combustion). On the whole, under the oxygen-enriched conditions, the overall combustion rate of volatile increases significantly. Compared with the two oxygen enrichment schemes of primary air oxygen enrichment and secondary air oxygen enrichment it can be seen that, when y<4.3 m, the combustion rate of volatile is higher in the oxygen-enriched condition of the primary air, which is caused by the larger contact area of the pulverized coal with the primary air when it enters the rotary kiln. When y>4.3 m, the combustion rate of volatile in the oxygen enrichment of the secondary air is slightly higher than that in the oxygen enrichment condition of the primary air. This is because the pulverized coal continuously spreads along the transverse direction during the longitudinal movement of the rotary kiln, thereby making more contact with the secondary air.
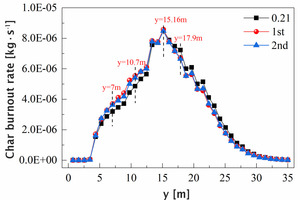
Figure 15 shows that before y=15.16 m, the combustion rate of char under oxygen-enriched conditions is somewhat higher than that under the air combustion condition, which indicates that oxygen-enriched combustion can accelerate the combustion rate of pulverized coal in the burning zone, and concentrate the heat to be released at the burning zone of the rotary kiln, thereby facilitating the calcination of clinker. Comparing the two oxygen-enriched conditions, it can be seen that when y<17.9 m, especially when 7 m<y<10.7 m, the combustion rate of char is higher in the oxygen enrichment condition of the primary air, which is beneficial to increase the temperature of the burning zone at the front end of the rotary kiln.
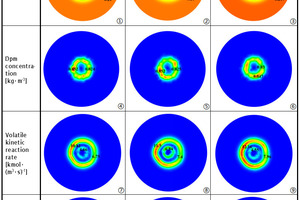
In order to further understand the effect of oxygen concentration on the combustion process of pulverized coal, at the position of the highest volatile combustion rate (Figure 14, y=8 m), the contours of O2 mole fraction, dpm concentration, volatile kinetic reaction rate and char burnout rate under three working conditions are shown in Figure 16. The combustion process of pulverized coal under air combustion conditions is shown in (1), (4), (7) and (10). It can be found that part of the pulverized coal (4) diffuses inward and reacts with O2 ((7) and (10)) which is called inner O2 in this paper (1) in the central zone. More pulverized coal (4) diffuses outward (in the direction to the secondary air) and reacts with the O2 ((7) and (10)) which is called outer O2 in this paper (1) brought in by the swirling air, axial air and secondary air in the outer area ((7) and (10)).
Obviously, there is more internal oxygen under the oxygen enrichment of primary air and more external oxygen under the oxygen enrichment of secondary air, which can be seen from (2) and (3)in Figure 16. Regardless of the oxygen enrichment scheme, the oxygen concentration in the flow field is higher than that in the air combustion scheme. Comparing the two oxygen-enriched conditions, it can be seen that the volatile combustion rate in the outer O2 zone is higher ((8) and (9)) when the secondary air is enriched, while the volatile combustion rate and char burnout rate in the inner O2 zone are both higher when the primary air is enriched, which is consistent with the results of Figures 14 and 15.
5.4 Combustion effect
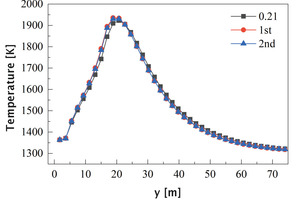
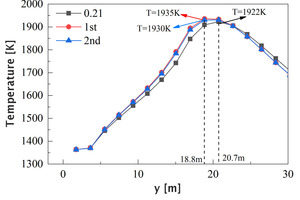
Figure 17 shows the average temperature curves of the section along the length of the kiln and a partial enlarged view. It can be seen that the temperature distribution curves under the three working conditions are all peak-shaped. Compared with the air combustion condition, the peak position in the oxygen-enriched condition is moved forward from 20.87 m to 18.8 m, which is due to the decrease of the secondary air velocity during the oxygen-enriched combustion (see Table 2). Before 20.7 m, the average temperature in the kiln during oxygen-enriched combustion is higher than that in the air combustion condition, and the increase of the average temperature by the oxygen-enriched primary air is more obvious, in which the peak temperature increased from 1922 K to 1935 K, an increase of 13 °C; after 22.6 m, the average temperature in the oxygen-enriched condition is slightly lower than that in the air combustion condition, which indicates that the combustion reaction process is more concentrated in the burning zone under the oxygen-enriched condition, which is beneficial to the calcination of clinker.
There are three types of heat transfer in rotary kiln: conduction, convection and radiation. In the burning zone, the flame transfers heat to the material in the form of radiation, and transfers the heat absorbed by the kiln skin to the contacting material in the form of conduction heat transfer. In this process, the radiation heat transfer accounts for 90% of the heat transfer in the burning zone [18]. There are many factors that affect radiation heat transfer in rotary kiln, such as flame temperature, radiant gas (CO2 and H2O) concentration, solid particles, etc.
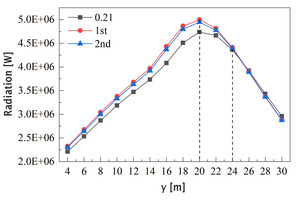
This rotary kiln model does not take into account the accumulation and movement of materials, and the radiation received by different walls surface along the kiln length is approximately equal to the radiation received by the materials, so we use the radiant heat received by the wall surface to study the effect of oxygen enriched combustion on clinker calcination. Figure 18 shows the curve of the average radiation received at the wall surface from 3 m to 31 m. As can be seen, the curve is peak-like in all three working conditions and peaks at 20 m, which is consistent with the peak position of the average temperature curve (Figure 17). In contrast, before 26 m, the radiant heat received at the wall surface in the oxygen-enriched condition is higher than that in the air combustion condition, and the highest value is found in the in the primary air enrichment scheme.
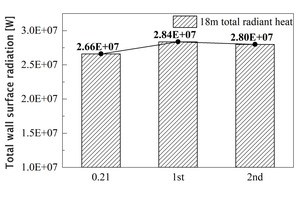
Under the actual air combustion conditions, the burning zone of this rotary kiln is generally located at 3 m-18 m. In order to study the effect of oxygen enrichment on the radiation received by the burning zone of the rotary kiln, the cumulative wall radiation from 0 m to 18 m is shown in Figure 19. It can be seen that the primary air oxygen enrichment scheme has the greatest improvement on the total radiation in the burning zone, and the total radiation increases from 2.66×107 W in air combustion condition to 2.84×107 W, an increase of about 6.76%.
5.5 NOx concentration
NOx produced by rotary kiln includes thermal NOx, instantaneous NOx and fuel NOx. Due to the high temperature in the kiln, the generation of thermal NOx is large. Its formation is closely related to temperature, and the reaction rate is slower when the temperature is lower than 1350 °C (1623 K), while the formation rate of NOx increases exponentially with the increase of temperature when the temperature is higher than 1500 °C (1773 K) [19].
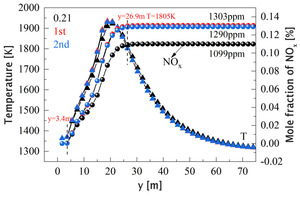
Figures 20-21 are the average mole fraction curves of NOx, O2, and CO2 along the length of the kiln, respectively. Combining Figure 11 and Figure 20, it can be seen that the generation position of NOx is consistent with that of the high temperature zone, and its average generation rate corresponds to the distribution interval of the local high temperature zone (Figure 11) and the average temperature increase rate (Figure 20). When y>3.4 m, volatile and char begin to burn, resulting in local high temperature, and NOx also begins to be generated. When y>26 m, the temperature in the high temperature zone begins to be lower than 1800 K, and after this position, NOx no longer increases (Figure 20).
Comparing the three working conditions, it can be seen that the amount of NOx generated in the oxygen-enriched condition is significantly higher than that in the air-assisted combustion condition. The amount of NOx generated when the primary air is enriched with oxygen is the largest, and the NOx content at the outlet increases from 1099 ppm to 1303 ppm, which is inseparable from the increase of kiln temperature and oxygen concentration when enriched with oxygen. In this case, more O2 was consumed and more CO2 was produced (Figure 21), indicating a more complete reaction, resulting in a higher average temperature in the burning zone (Figure 20), and thus more NOx production.
6 Conclusions
Aiming at a real rotary kiln, numerical simulation studies were carried out for air combustion, primary air enriched oxygen, and secondary air enriched oxygen conditions, respectively. The optimal oxygen enrichment method was found by comparing the flow fields, high temperature zone coverage shape, average temperature distribution, volatile combustion rate, char combustion rate and NOx concentration field in the rotary kiln. The simulation results show that:
There is no obvious change in the flow fields before and after oxygen enrichment. In the head of the burner region, a central recirculation zone and an external recirculation zone are formed due to the entrainment phenomenon, which is beneficial to the pulverized coal mixing and combustion.
he high temperature zone is “willow leaf-like” before and after oxygen enrichment. When oxygen enrichment was used, in the burning zone of the rotary kiln, the combustion speed of the volatiles and char in the pulverized coal is accelerated, resulting in an increase in the overall temperature at the front end of the rotary kiln. The oxygen enrichment in the primary air has the most obvious improvement on the average temperature, in which the peak position is advanced by 2 m, and the extreme value of the average temperature is increased by 13 °C, which is beneficial to the clinker calcination.
When oxygen enrichment was used, the total radiation received by the wall surface of the rotary kiln increased, indicating that when enriched with oxygen, the temperature of the burning zone and the amount of radiation received by the material can be increased, thereby saving energy and increasing production. The improvement of the total radiation is the largest when the primary air was enriched with oxygen, and the total radiation received by the wall from 3 m to -18 m increased by about 6.76%.
The generation of NOx increased significantly when the oxygen was enriched, and the increase was the largest when the primary air was enriched with oxygen, in which the NOx concentration at the outlet increased from 1099 ppm to 1303 ppm.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.