Evolution of heat during the synthesis of hydrosilicates in aerated concrete
The heat evolved during the synthesis of 11.3 Å tobermorite from lime and quartz is 125 kJ/kg and the synthesis of xonolite is 50 kJ/kg, which raises the pressure and temperature in the autoclave. Recommendations are given below for controlling the composition of the hydrate and for reducing the consumption of steam during the autoclaving.
1 Introduction
1 Introduction
The following values were assumed for the reaction enthalpy and also for the heat capacity of tobermorite and its intermediate phases:
Data [1] in:
The molar standard entropy in kcal/Mol (or kJ/Mol)
2 Calculations
This means that the theoretical result for the heat evolution for tobermorite agrees with the values from Babuschkin [1].
The rise in temperature in the autoclave can now be determined, for which the specific heat capacity of tobermorite is calculated as follows:
In this case m = 1 kg, which gives a temperature rise of ∆t 30,1 1,33 = 22,6°. The heat capacity of tobermorite is slightly higher than that of the starting components so ∆t = 23 °C is used for the calculation.
The heat evolved during the synthesis of xonolite is calculated in a similar way:
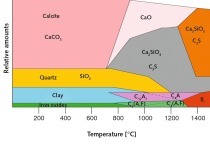
Rahimbaev [2] shows that the change in enthalpy under isothermal conditions is accompanied by slight change in the evolution of heat in the bonding system. From this it emerges that the evolution of heat during the synthesis of 11.3 Å tobermorite, such as occurs in aerated concrete during hardening in the autoclave, contributes much more than that of xonolite.
The evolution of heat during the synthesis of tobermorite in aerated concrete causes a local temperature rise within the material by 22-23 °C.
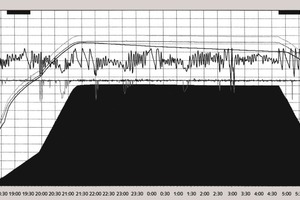
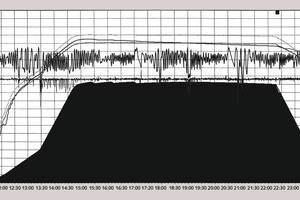
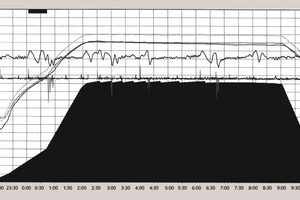
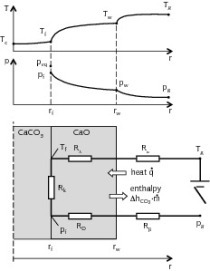
3 Graphical representation
Analysis of the curves that characterize the process occurring in the autoclave shows the following:
During the autoclaving of the product with a density D 600 the steam pressure remains stable at 1.1 MPa after the operating point has been reached.
During the autoclaving of the aerated concrete products with densities of D 400 and 500 the steam pressure rises by 0.025-0.03 MPa after the operating point has been reached. This then triggers the automatic control system for the steam pressure in the autoclave and switches off the steam supply so that after a few minutes the pressure falls back to the set value of 1.1 MPa. The pressure in the autoclave then rises until the automatic control system is actuated again.
The number of on-off cycles of the automatic control system varies between 2 and 7 while the intervals between the cycles between 15 and 20 minutes may be as much as 150 minutes. An entire cycle lasts between 2 and 3.5 hours.
The number of on-off cycles falls during the course of time. Although the first two disconnections occur after 15-20 minutes the last two will occur 40-50 minutes after the previous one.
The frequency and amplitude of the switching on and off of the steam supply depend on the quantity of lime and the aluminium powder or aluminium paste. This shows indirectly how the rise in steam pressure in the autoclave is determined not only by the synthesis of the silicate hydrates but also by the aluminate hydrate phases. However, the contribution of the aluminate hydrate phases in the overall heat balance is apparently relatively small.
A rising enthalpy displaces the reaction in the direction of xonolite while a rising pressure or entropy causes a displacement in the direction of tobermorite formation. The energy factor clearly exerts an dominant influence, so an intensification of the conditions in the autoclave favours the formation of xonolite in the CaO-SiO2-H2O system although it is transformed back into tobermorite again if the concrete is cooled under conditions of high humidity [3].
4 Conclusion
For aerated concrete production it is therefore recommended that immediately after removal from the autoclave the product should be dried to a residual moisture content of not more than 20 %, which also contributes to stabilization of the beneficial phase composition. Because of the above-mentioned results from the autoclaving of aerated concretes with densities D 400 and 500 the steam pressure should be reduced by 0.025-0.03 MPa for the first 3–4 hours after the correct operating point has been reached. The evolution of heat during the synthesis of silicon and aluminate hydrates also lowers the energy costs by a few percent.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.