Why CO2 matters – advances in a new class of cement
1 Introduction
The blanket effect of greenhouse gas emissions is increasing the temperature of the earth’s surface. As the concentration of greenhouse gases increase in the atmosphere, the earth’s surface gets warmer [1]. Carbon dioxide (CO₂) constitutes roughly 77% of total global greenhouse gas emissions in the atmosphere, and for most of the last 800,000 years, CO₂ levels have stayed below 300 ppm [2]. With the onset of the industrial age, CO₂ concentrations have increased steadily, reaching 335 ppm in 1980 [3]. As of February 2013, CO₂ concentration levels reached 397 ppm [3]. According...
1 Introduction
The blanket effect of greenhouse gas emissions is increasing the temperature of the earth’s surface. As the concentration of greenhouse gases increase in the atmosphere, the earth’s surface gets warmer [1]. Carbon dioxide (CO₂) constitutes roughly 77% of total global greenhouse gas emissions in the atmosphere, and for most of the last 800,000 years, CO₂ levels have stayed below 300 ppm [2]. With the onset of the industrial age, CO₂ concentrations have increased steadily, reaching 335 ppm in 1980 [3]. As of February 2013, CO₂ concentration levels reached 397 ppm [3]. According to the Intergovernmental Panel on Climate Change (IPCC), CO₂ concentration must remain below 450 ppm in order to maintain a global temperature increase of less than 3 °C [1].
2 Cement, concrete and CO2
According to a 2005 study by the World Resources Institute (WRI) on greenhouse gas emissions by major industries, the cement industry is responsible for 3.8 % of the total global greenhouse gas emissions, which is equivalent to 5-7% of industrial CO₂ emissions [4]. Likewise, other studies show that the cement industry accounts for about 5 % of global anthropogenic carbon dioxide emissions [5].
While Portland cement concrete has a very low carbon footprint compared to other industrial products, Portland cement itself is a significant CO₂ emitter because of its high-volume production. Concrete is the world’s second most utilized substance, exceeded only by the consumption of water. Over 30 Bt of Portland cement concrete (PCC), containing approximately 3 Bt of Portland cement, are manufactured and used every year. The US Geological Survey reports that the world’s cement production was 3.4 Bt in 2011 [6].
The International Energy Agency (IEA) has set a target for each industry to decrease their CO₂ emissions. According to this plan, the cement industry must reduce its CO₂ emissions to 1.55 Gt by 2050 from 2.0 Gt in 2007 [7]. However, the cement production in 2050 is expected to increase by 43 to 73%. The estimated production in 2050 will be between 3.7 and 4.4 Bt [8]. In order to meet these specified goals, the CO₂ emission per ton of cement must be reduced from 0.8 t to between 0.35 to 0.42 t.
Portland cement clinker is made by burning a mixture of ground calcareous material, such as limestone or chalk, and siliceous material, such as sand, clay, or materials of similar composition, in a rotary kiln at a sintering temperature of 1450°C. This process yields clinker nodules, which are further ground with the addition of about 5 % gypsum to produce the finished cement.
During cement production, CO₂ emissions come from two main sources:
CO₂ released from the decomposition of limestone, CaCO3 (s), which produces lime, CaO (s), and CO₂ (g); and
CO₂ generated during the combustion of fossil fuels to heat the kiln to the required sintering temperature.
A third minor source of CO₂ emission is from electricity generation and the transportation of materials.
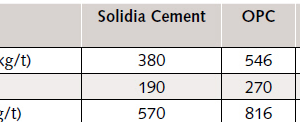
A typical Portland cement clinker requires 70 % CaO by weight. During calcination of the limestone to produce the required amount of CaO, ~ 546 kg of CO₂ gas is produced per ton of cement clinker [9]. A significant amount of CO₂ is emitted due to fuel combustion, and it depends on the type and the efficiency of the kiln. For example, a five stage, pre-heater kiln with pre-calciner has the highest efficiency, 58 %, and emits ~ 270 kg CO₂ per ton of clinker during combustion [10]. This number can be as high as 600 kg of CO₂ per ton of clinker with older, wet processing which has an efficiency of 26-30 % [10]. As a result, the total CO₂ emission per ton of Portland cement clinker varies from ~ 816 kg to 1.1 t depending on the type of kiln and process used, in which, more than half ton of the emission comes from the decomposition of limestone.
To reduce CO₂ emissions, the cement industry has applied several strategies, which include the use of energy-efficient process technologies, alternative fuels, and supplementary cementitious materials. These approaches may help reduce emissions moderately, but there is a clear need for a transformative innovation to meet the CO₂ emissions goal set by the IEA of 1.55 Gt by 2050.
3 Strategies for decreasing
CO2 emissions
The cement industry has taken several measures to decrease its CO₂ emissions, including the modernization and implementation of energy-efficient production technologies combined with the use of alternative fuels. With these approaches, CO₂ emission from fuel consumption can be decreased by 55 %. Another approach is the reduction of the clinker factor in cement by adding supplementary cementitious materials (SCMs). The reduction in clinker factor is achieved by co-grinding cement clinker with SCMs, such as fly ash, slag, natural pozzolanic materials, and fillers, such as limestone. The world average for clinker factor decreased from 83 % to 78 % between 1990 and 2006 [11]. However, even the combined effect of these initiatives is likely to fall far short of the IEA roadmap goals. For example, as of today, if all of the cement in the world was produced in the most energy-efficient kilns and if the clinker factor in the cement were reduced to 78 %, the amount of CO₂ emitted per ton of finished cement would be ~ 0.64 t as compared to the target of 0.35 to 0.42 t.
The remaining options for the cement industry are to find either a cost-effective carbon capture and storage (CCS) technology and/or to explore different cement chemistries that require less CaO.
Existing CCS technologies are expensive and would add significant additional costs to Portland cement manufacturing. Belite cement is an example of a cement chemistry that requires less CaO. In Belite cement, the CaO content is decreased from 70 % to 64 % [10]. As a result, the CO₂ emissions from raw materials drop from 546 kg to 501 kg per ton of clinker [10]. Additionally, Belite cement manufacturing requires ~ 20 % less energy compared to Portland cement [10]. For comparison, if Belite cement is produced in a five stage pre-heater kiln with pre-calciner, the overall CO₂ emissions per ton of clinker would decrease from 816 kg to 719 kg.
The difficulty in further lowering the lime content in cement stems from the curing of Portland cement, which relies on hydration of calcium rich phases in the presence of water. Once the calcium content is decreased, the lack of high early strength becomes an issue. Even though there is some CO₂ savings (approximately 12 %) with Belite cements, the performance disadvantage of Belite cement concrete limits the viability of this approach.
In addition to initiatives undertaken by the Portland cement industry, some technology companies have proposed radically different solutions. These solutions included bubbling CO₂ into sea brine to precipitate cementitious calcium magnesium carbonate compounds while increasing the pH of the solution by adding basic chemicals, and using olivine to produce magnesium based cements. The main disadvantages of these technologies are the high adoption costs along with some technological barriers.
4 A new class of cement
Cement plants, which are typically built near limestone quarries, cost around US$ 300-400 million to build. There are approximately 110 cement plants in the United States, making the total capital investment of the US cement industry approximately US$ 30 to US$ 40 billion.
Solutions to the cement industry’s CO₂ goals that require drastically different raw materials and/or equipment would be prohibitively expensive. Ideally, a successful solution would incur minimal adoption costs. It would be based on the same equipment and raw materials. In addition, the solution must be widely accepted, and the product must have equal or better performance.
Solidia Technologies has developed a new class of cement with a very low-carbon footprint that can be produced in any cement plant from the existing raw materials. The curing of Solidia Cement™ relies on carbonation rather than hydration. Unlike hydration, carbonation does not require calcium rich species. This unique feature enables new chemistries for cement production with low CaO demand. The CO₂ savings that Solidia Technologies offers is not limited to cement production; during the curing of Solidia Cement, a significant amount of CO₂ is consumed due to the carbonation reaction of calcium silicate phases.
5 Solidia cement chemistry and CO2 savings
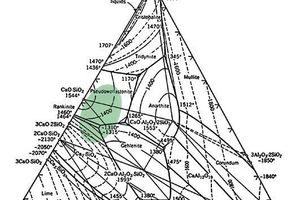
Solidia Cement is based on a mixture of calcium silicate and calcium silicate aluminate compounds with the overall molar CaO to SiO2 ratio of ~ 1. The calcium silicate phases are mainly pseudo-wollastonite along with some rankinite and larnite. Impurities such as Al, Mg and Fe form complex melilite group of minerals. The approximate compositional range of Solidia Cement is shown in CaO-SiO2-Al2O3 ternary phase diagram (Fig. 1). [12]
While 70 % CaO is necessary in Portland cement clinker, Solidia Cement requires only approximately 45 %. This results in an approximate 30 % reduction in CO₂ emissions coming from the raw materials. Solidia Cement is synthesized at around 1200°C, which is roughly ~ 250°C lower than the Portland cement sintering temperature. The lower kiln temperature yields approximately 30 % less CO₂ emissions and significant savings in fuel costs. (See the white paper, Solidia Cement™, for more details on its chemistry [13]).
Overall, approximately 816 kg of CO₂ are emitted from a cement kiln per ton of Portland cement clinker. The production of Solidia Cement clinker (Fig. 2 and 3) can reduce this quantity by approximately 30 %. The overall CO₂ emissions for making one ton of Solidia Cement clinker in a five-stage pre-heater kiln with pre-calciner is estimated to be around 570 kg, of which 380 kg derives from the raw materials and approximately 190 kg from the fuel combustion. This comparison is tabulated in Table 1.
Solidia Cement can also be used with SCMs and filler materials to decrease the clinker factor to provide additional savings.
5.1 Curing of solidia cement concrete and additional CO2 savings
The forming of concrete products made from Solidia Cement is the same as forming of Portland cement products. The formulations may be slightly different to adjust the workability and the final property of the products.
After forming, the concrete products made with Solidia Cement are placed in a high-concentration CO₂ environment. This environment can be achieved by processes as simple as placing a sealed tarp over the concrete part and pumping in CO₂ to achieve 60-90 % concentration. Like Portland cement concrete curing, heat may be applied to speed up the curing process. Since there is no ettringite formation during Solidia Cement curing, the curing temperature can be higher than 60°C if needed. The CO₂ used during the curing process is derived from industrial by-product, which is captured, packaged and delivered to the concrete manufacturing site by industrial gas suppliers.
The CO₂-curing process is a counter-diffusion process in which the CO₂ molecules replace water molecules inside the pores of the concrete and react with the calcium silicate phases to precipitate calcium carbonate and silica. The carbonation reaction is an exothermic process, releasing about 87 kJ/mol of heat during curing. The heat is dissipated through the evaporation process of the water that is used in the concrete formulation. The curing time varies from a couple of hours to one day depending on the sample size and geometry. A typical concrete made from 16 wt% Solidia Cement will sequester about 5 wt% of CO₂ of its total mass. When one ton of Solidia Cement is used, the amount of CO₂ consumed by the concrete will be around 250-300 kg. The properties of concrete products made from Solidia Cement are similar to those of Portland cement concrete products (Fig. 4) and is discussed in a separate paper [14].
The CO₂ emissions discussed above are for comparison purposes only. The percentages and actual values may vary from plant to plant depending on the type of the kiln and process used. For simplicity of the estimations, the CO₂ emitted during transportation of materials, milling of cement and curing of concrete products are assumed to be the same for Portland and Solidia Cement products, and are not included in the calculations. Nevertheless, the combined CO₂ savings during the synthesis and curing of Solidia Cement will decrease the CO₂ emissions of the cement industry significantly. It will enable the cement industry to reach or get very close to the IEA’s 2050 goals without requiring any significant changes to existing production processes and equipment.

Überschrift Bezahlschranke (EN)
tab ZKG KOMBI EN
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
tab ZKG KOMBI Study test
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.
This is a trial offer for programming testing only. It does not entitle you to a valid subscription and is intended purely for testing purposes. Please do not follow this process.